Emf Of Standard Hydrogen Electrode . in electrochemistry, standard electrode potential, or , is a measure of the reducing power of any element or compound. the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic or voltaic cell. It has a cell potential of 0.00v, measured. standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. What is a standard hydrogen electrode? the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework.
from www.numerade.com
What is a standard hydrogen electrode? the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic or voltaic cell. It has a cell potential of 0.00v, measured. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. in electrochemistry, standard electrode potential, or , is a measure of the reducing power of any element or compound. standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the.
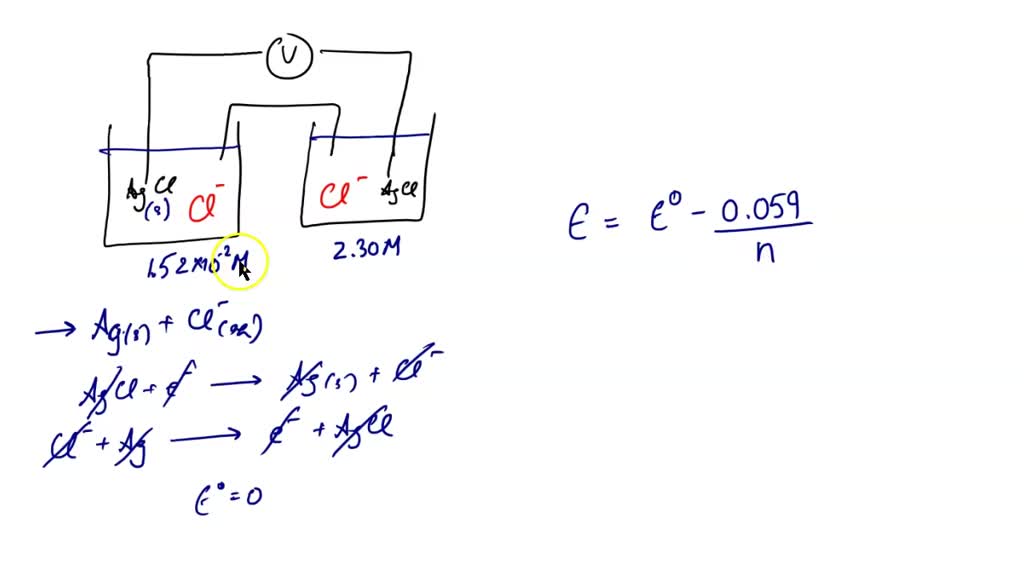
SOLVED A voltage cell is set up from a chlorine and a hydrogen
Emf Of Standard Hydrogen Electrode the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic or voltaic cell. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. in electrochemistry, standard electrode potential, or , is a measure of the reducing power of any element or compound. It has a cell potential of 0.00v, measured. the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic or voltaic cell. the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. What is a standard hydrogen electrode?
From scienceinfo.com
What is Standard Hydrogen Electrode? Emf Of Standard Hydrogen Electrode tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic or voltaic cell. What is a standard hydrogen electrode? The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be. Emf Of Standard Hydrogen Electrode.
From cider.uoregon.edu
Standard Hydrogen Electrode (SHE) Simulation and Animation AACT CIDER Emf Of Standard Hydrogen Electrode in electrochemistry, standard electrode potential, or , is a measure of the reducing power of any element or compound. What is a standard hydrogen electrode? the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. tabulating all electrode potentials with respect to the same. Emf Of Standard Hydrogen Electrode.
From www.youtube.com
Standard Hydrogen Electrode (SHE)/ Primary reference electrode Gas Emf Of Standard Hydrogen Electrode It has a cell potential of 0.00v, measured. the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. What is a standard hydrogen electrode? tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. The standard hydrogen electrode is. Emf Of Standard Hydrogen Electrode.
From www.bartleby.com
Answered What is the effect on the emf of the… bartleby Emf Of Standard Hydrogen Electrode the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic or voltaic cell. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. the emf measured. Emf Of Standard Hydrogen Electrode.
From askfilo.com
At 298 K the emf of the cell, standard hydrogen electrode II second half.. Emf Of Standard Hydrogen Electrode What is a standard hydrogen electrode? tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. in electrochemistry, standard electrode potential, or , is a measure of the reducing power of any element or compound. the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode. Emf Of Standard Hydrogen Electrode.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID541675 Emf Of Standard Hydrogen Electrode The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. tabulating all electrode potentials with respect to the same standard electrode provides a practical working. Emf Of Standard Hydrogen Electrode.
From mmerevise.co.uk
Calculating the EMF Emf Of Standard Hydrogen Electrode the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic or voltaic cell. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to. Emf Of Standard Hydrogen Electrode.
From hxeitqhyu.blob.core.windows.net
Standard Hydrogen Electrode Images at Rose Kennedy blog Emf Of Standard Hydrogen Electrode It has a cell potential of 0.00v, measured. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. What is a standard hydrogen electrode? The standard hydrogen electrode is. Emf Of Standard Hydrogen Electrode.
From askfilo.com
At 298 K the emf of the cell, standard hydrogen electrode Il second half.. Emf Of Standard Hydrogen Electrode the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic or voltaic cell. What is a standard hydrogen electrode? the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. tabulating all electrode potentials with respect to the same standard. Emf Of Standard Hydrogen Electrode.
From cider.uoregon.edu
Standard Hydrogen Electrode Demonstration and AACT Simulation CIDER Emf Of Standard Hydrogen Electrode in electrochemistry, standard electrode potential, or , is a measure of the reducing power of any element or compound. standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. It has a. Emf Of Standard Hydrogen Electrode.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Emf Of Standard Hydrogen Electrode standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. It has a cell potential of 0.00v, measured. in electrochemistry, standard electrode potential, or , is a measure of the reducing power of any element or compound. the electromotive force (emf) is the maximum potential difference between two. Emf Of Standard Hydrogen Electrode.
From www.vrogue.co
Hydrogen Standard Hydrogen Electrode vrogue.co Emf Of Standard Hydrogen Electrode The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. What is a standard hydrogen electrode? It has a cell potential of 0.00v, measured. the. Emf Of Standard Hydrogen Electrode.
From byjus.com
What is standard hydrogen electrode? Emf Of Standard Hydrogen Electrode What is a standard hydrogen electrode? It has a cell potential of 0.00v, measured. in electrochemistry, standard electrode potential, or , is a measure of the reducing power of any element or compound. standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. the emf measured when a. Emf Of Standard Hydrogen Electrode.
From www.youtube.com
Calculation of pH with emf l Standard Hydrogen electrode l Calomel l Emf Of Standard Hydrogen Electrode The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. in electrochemistry, standard electrode potential, or , is a measure of the reducing power of any element or compound. the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic. Emf Of Standard Hydrogen Electrode.
From saylordotorg.github.io
Electrochemistry Emf Of Standard Hydrogen Electrode standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic or voltaic cell. What is a standard hydrogen electrode? the emf measured when a metal / metal ion electrode is coupled to a. Emf Of Standard Hydrogen Electrode.
From www.youtube.com
(L14) SHE(Standard Hydrogen Electrode) for EMF Calculation NEET JEE Emf Of Standard Hydrogen Electrode the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic or voltaic cell. It has a cell potential of 0.00v, measured. in electrochemistry, standard electrode potential, or , is a measure of the reducing power of any element or compound. The standard hydrogen electrode is often abbreviated to she, and its standard electrode. Emf Of Standard Hydrogen Electrode.
From www.youtube.com
Electrochemistry Part 3 EMF of the cell & Standard Hydrogen Emf Of Standard Hydrogen Electrode tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic or voltaic cell. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k.. Emf Of Standard Hydrogen Electrode.
From www.youtube.com
Standard Hydrogen Electrode Demonstration YouTube Emf Of Standard Hydrogen Electrode tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0. Emf Of Standard Hydrogen Electrode.
From askfilo.com
At 298 K the emf of the cell, standard hydrogen electrode II second half.. Emf Of Standard Hydrogen Electrode in electrochemistry, standard electrode potential, or , is a measure of the reducing power of any element or compound. What is a standard hydrogen electrode? the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. the electromotive force (emf) is the maximum potential difference. Emf Of Standard Hydrogen Electrode.
From cider.uoregon.edu
Standard Hydrogen Electrode Demonstration and AACT Simulation CIDER Emf Of Standard Hydrogen Electrode The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. It has a cell potential of 0.00v, measured. What is a standard. Emf Of Standard Hydrogen Electrode.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Emf Of Standard Hydrogen Electrode tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic or voltaic cell. standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. the emf measured. Emf Of Standard Hydrogen Electrode.
From exowggogj.blob.core.windows.net
What Is The Emf Of Hydrogen Electrode at Lewis Hunter blog Emf Of Standard Hydrogen Electrode tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. It has a cell potential of 0.00v, measured. What is a standard hydrogen electrode? the electromotive force (emf). Emf Of Standard Hydrogen Electrode.
From exowggogj.blob.core.windows.net
What Is The Emf Of Hydrogen Electrode at Lewis Hunter blog Emf Of Standard Hydrogen Electrode The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. tabulating all electrode potentials with respect to the same standard electrode provides a practical working. Emf Of Standard Hydrogen Electrode.
From www.numerade.com
SOLVED A voltage cell is set up from a chlorine and a hydrogen Emf Of Standard Hydrogen Electrode in electrochemistry, standard electrode potential, or , is a measure of the reducing power of any element or compound. It has a cell potential of 0.00v, measured. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. the emf measured when a metal /. Emf Of Standard Hydrogen Electrode.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Emf Of Standard Hydrogen Electrode What is a standard hydrogen electrode? standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. in electrochemistry, standard electrode potential, or , is a measure of the reducing power of any element or compound. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential. Emf Of Standard Hydrogen Electrode.
From www.youtube.com
20.10 calculating emf with a standard hydrogen electrode YouTube Emf Of Standard Hydrogen Electrode standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of. Emf Of Standard Hydrogen Electrode.
From hxeiukiwg.blob.core.windows.net
Standard Hydrogen Electrode Voltage at Wanda Estes blog Emf Of Standard Hydrogen Electrode What is a standard hydrogen electrode? the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic or voltaic cell. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. the emf measured when a metal / metal ion electrode. Emf Of Standard Hydrogen Electrode.
From issuu.com
standard hydrogen electrode by norainihamzah9 Issuu Emf Of Standard Hydrogen Electrode What is a standard hydrogen electrode? standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic or voltaic cell. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is. Emf Of Standard Hydrogen Electrode.
From www.vrogue.co
Hydrogen Standard Hydrogen Electrode vrogue.co Emf Of Standard Hydrogen Electrode the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0. Emf Of Standard Hydrogen Electrode.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Emf Of Standard Hydrogen Electrode in electrochemistry, standard electrode potential, or , is a measure of the reducing power of any element or compound. standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. What is a. Emf Of Standard Hydrogen Electrode.
From hxeiukiwg.blob.core.windows.net
Standard Hydrogen Electrode Voltage at Wanda Estes blog Emf Of Standard Hydrogen Electrode in electrochemistry, standard electrode potential, or , is a measure of the reducing power of any element or compound. standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. It has a cell potential of 0.00v, measured. the electromotive force (emf) is the maximum potential difference between two. Emf Of Standard Hydrogen Electrode.
From www.shutterstock.com
Diagramme standard d'électrode à hydrogène. Illustration image Emf Of Standard Hydrogen Electrode in electrochemistry, standard electrode potential, or , is a measure of the reducing power of any element or compound. the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic or voltaic cell. the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is. Emf Of Standard Hydrogen Electrode.
From www.researchgate.net
Comparison of the voltage vs. standard hydrogen electrode (SHE Emf Of Standard Hydrogen Electrode the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. in electrochemistry, standard electrode potential, or , is a measure of. Emf Of Standard Hydrogen Electrode.
From askfilo.com
haifcell K the emf of the cell, standard hydrogen electrode ll second re.. Emf Of Standard Hydrogen Electrode the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. It has a cell potential of 0.00v, measured. the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic or voltaic cell. The standard hydrogen electrode is often abbreviated to she,. Emf Of Standard Hydrogen Electrode.
From www.youtube.com
Electrochemistry 13 Standard Hydrogen Electrode Construction Emf Of Standard Hydrogen Electrode What is a standard hydrogen electrode? standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. the emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. in electrochemistry, standard electrode potential, or , is a. Emf Of Standard Hydrogen Electrode.