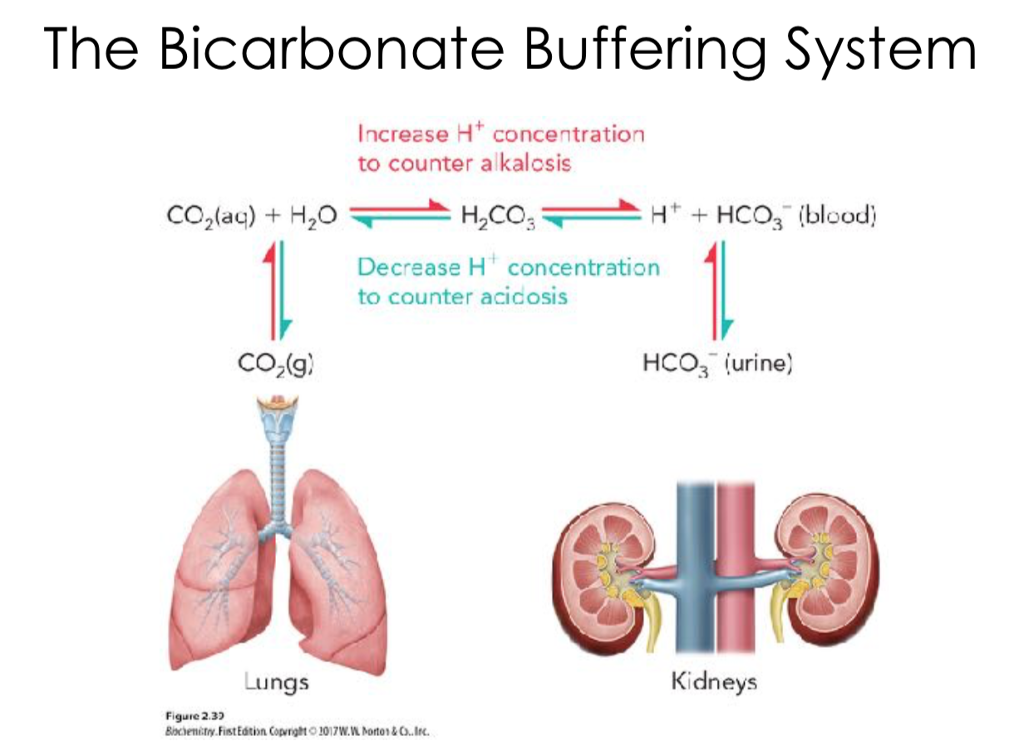
Carbonic Acid Buffer System In Human Blood . Learn how the bicarbonate buffer system, respiration and kidneys regulate blood ph within a narrow range of 7.35 to 7.45. Carbonic anhydrase is in red blood cells, renal tubules, gastric mucosa, and pancreatic cells. Other buffer systems in the human body include the phosphate buffer. The bicarbonate buffer system is the largest. Learn how carbonic acid and bicarbonate anion form a buffer system in human blood to maintain ph between 7.35 and 7.45. Find out how the respiratory and. In this system, gaseous metabolic waste carbon dioxide reacts. Learn how the bicarbonate buffer system maintains ph in the blood and duodenum by balancing carbonic acid, bicarbonate ion, and.
from www.chegg.com
Other buffer systems in the human body include the phosphate buffer. Learn how carbonic acid and bicarbonate anion form a buffer system in human blood to maintain ph between 7.35 and 7.45. Learn how the bicarbonate buffer system maintains ph in the blood and duodenum by balancing carbonic acid, bicarbonate ion, and. The bicarbonate buffer system is the largest. Carbonic anhydrase is in red blood cells, renal tubules, gastric mucosa, and pancreatic cells. Learn how the bicarbonate buffer system, respiration and kidneys regulate blood ph within a narrow range of 7.35 to 7.45. Find out how the respiratory and. In this system, gaseous metabolic waste carbon dioxide reacts.
Solved The Bicarbonate Buffering System Increase H
Carbonic Acid Buffer System In Human Blood Find out how the respiratory and. Find out how the respiratory and. Learn how carbonic acid and bicarbonate anion form a buffer system in human blood to maintain ph between 7.35 and 7.45. Carbonic anhydrase is in red blood cells, renal tubules, gastric mucosa, and pancreatic cells. The bicarbonate buffer system is the largest. Learn how the bicarbonate buffer system, respiration and kidneys regulate blood ph within a narrow range of 7.35 to 7.45. Learn how the bicarbonate buffer system maintains ph in the blood and duodenum by balancing carbonic acid, bicarbonate ion, and. In this system, gaseous metabolic waste carbon dioxide reacts. Other buffer systems in the human body include the phosphate buffer.
From slideplayer.com
Introduction into biochemistry ppt download Carbonic Acid Buffer System In Human Blood Carbonic anhydrase is in red blood cells, renal tubules, gastric mucosa, and pancreatic cells. Other buffer systems in the human body include the phosphate buffer. Find out how the respiratory and. Learn how carbonic acid and bicarbonate anion form a buffer system in human blood to maintain ph between 7.35 and 7.45. Learn how the bicarbonate buffer system, respiration and. Carbonic Acid Buffer System In Human Blood.
From www.pinterest.com
Physiology Blood Buffer System Behrouz Human body facts Carbonic Acid Buffer System In Human Blood Learn how the bicarbonate buffer system maintains ph in the blood and duodenum by balancing carbonic acid, bicarbonate ion, and. Learn how carbonic acid and bicarbonate anion form a buffer system in human blood to maintain ph between 7.35 and 7.45. Learn how the bicarbonate buffer system, respiration and kidneys regulate blood ph within a narrow range of 7.35 to. Carbonic Acid Buffer System In Human Blood.
From www.slideshare.net
11 acid base regulation Carbonic Acid Buffer System In Human Blood Learn how the bicarbonate buffer system maintains ph in the blood and duodenum by balancing carbonic acid, bicarbonate ion, and. The bicarbonate buffer system is the largest. Learn how the bicarbonate buffer system, respiration and kidneys regulate blood ph within a narrow range of 7.35 to 7.45. In this system, gaseous metabolic waste carbon dioxide reacts. Find out how the. Carbonic Acid Buffer System In Human Blood.
From www.slideserve.com
PPT Chapter 27 Fluid, Electrolyte and Acidbase Balance PowerPoint Carbonic Acid Buffer System In Human Blood Carbonic anhydrase is in red blood cells, renal tubules, gastric mucosa, and pancreatic cells. Learn how the bicarbonate buffer system maintains ph in the blood and duodenum by balancing carbonic acid, bicarbonate ion, and. Learn how the bicarbonate buffer system, respiration and kidneys regulate blood ph within a narrow range of 7.35 to 7.45. Find out how the respiratory and.. Carbonic Acid Buffer System In Human Blood.
From www.pinterest.com
Image result for bicarbonate buffer system Medical student motivation Carbonic Acid Buffer System In Human Blood Learn how the bicarbonate buffer system, respiration and kidneys regulate blood ph within a narrow range of 7.35 to 7.45. Other buffer systems in the human body include the phosphate buffer. Carbonic anhydrase is in red blood cells, renal tubules, gastric mucosa, and pancreatic cells. Find out how the respiratory and. The bicarbonate buffer system is the largest. In this. Carbonic Acid Buffer System In Human Blood.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Carbonic Acid Buffer System In Human Blood Learn how the bicarbonate buffer system, respiration and kidneys regulate blood ph within a narrow range of 7.35 to 7.45. In this system, gaseous metabolic waste carbon dioxide reacts. Other buffer systems in the human body include the phosphate buffer. Learn how carbonic acid and bicarbonate anion form a buffer system in human blood to maintain ph between 7.35 and. Carbonic Acid Buffer System In Human Blood.
From www.slideserve.com
PPT Carbonic AcidBicarbonate Buffering System PowerPoint Carbonic Acid Buffer System In Human Blood Find out how the respiratory and. Other buffer systems in the human body include the phosphate buffer. Learn how the bicarbonate buffer system, respiration and kidneys regulate blood ph within a narrow range of 7.35 to 7.45. The bicarbonate buffer system is the largest. Carbonic anhydrase is in red blood cells, renal tubules, gastric mucosa, and pancreatic cells. Learn how. Carbonic Acid Buffer System In Human Blood.
From reverieranger.blogspot.com
How Does The Carbonic Acidbicarbonate Buffer System Maintain Blood Ph Carbonic Acid Buffer System In Human Blood Learn how the bicarbonate buffer system maintains ph in the blood and duodenum by balancing carbonic acid, bicarbonate ion, and. The bicarbonate buffer system is the largest. In this system, gaseous metabolic waste carbon dioxide reacts. Other buffer systems in the human body include the phosphate buffer. Learn how the bicarbonate buffer system, respiration and kidneys regulate blood ph within. Carbonic Acid Buffer System In Human Blood.
From www.chegg.com
Solved Carbonic acid in the blood forms an important buffer Carbonic Acid Buffer System In Human Blood The bicarbonate buffer system is the largest. Learn how carbonic acid and bicarbonate anion form a buffer system in human blood to maintain ph between 7.35 and 7.45. Learn how the bicarbonate buffer system maintains ph in the blood and duodenum by balancing carbonic acid, bicarbonate ion, and. Learn how the bicarbonate buffer system, respiration and kidneys regulate blood ph. Carbonic Acid Buffer System In Human Blood.
From www.visionlearning.com
Acids and Bases II Biology Visionlearning Carbonic Acid Buffer System In Human Blood In this system, gaseous metabolic waste carbon dioxide reacts. The bicarbonate buffer system is the largest. Learn how carbonic acid and bicarbonate anion form a buffer system in human blood to maintain ph between 7.35 and 7.45. Learn how the bicarbonate buffer system maintains ph in the blood and duodenum by balancing carbonic acid, bicarbonate ion, and. Carbonic anhydrase is. Carbonic Acid Buffer System In Human Blood.
From www.slideserve.com
PPT Chapter 27, part 2 PowerPoint Presentation, free download ID Carbonic Acid Buffer System In Human Blood Find out how the respiratory and. The bicarbonate buffer system is the largest. Learn how the bicarbonate buffer system maintains ph in the blood and duodenum by balancing carbonic acid, bicarbonate ion, and. Carbonic anhydrase is in red blood cells, renal tubules, gastric mucosa, and pancreatic cells. In this system, gaseous metabolic waste carbon dioxide reacts. Other buffer systems in. Carbonic Acid Buffer System In Human Blood.
From www.reagent.co.uk
How Do Biological Buffers Work? The Science Blog Carbonic Acid Buffer System In Human Blood Learn how carbonic acid and bicarbonate anion form a buffer system in human blood to maintain ph between 7.35 and 7.45. In this system, gaseous metabolic waste carbon dioxide reacts. Carbonic anhydrase is in red blood cells, renal tubules, gastric mucosa, and pancreatic cells. Learn how the bicarbonate buffer system, respiration and kidneys regulate blood ph within a narrow range. Carbonic Acid Buffer System In Human Blood.
From philschatz.com
AcidBase Balance · Anatomy and Physiology Carbonic Acid Buffer System In Human Blood In this system, gaseous metabolic waste carbon dioxide reacts. The bicarbonate buffer system is the largest. Find out how the respiratory and. Learn how carbonic acid and bicarbonate anion form a buffer system in human blood to maintain ph between 7.35 and 7.45. Carbonic anhydrase is in red blood cells, renal tubules, gastric mucosa, and pancreatic cells. Other buffer systems. Carbonic Acid Buffer System In Human Blood.
From beta.learner.org
The Buffer System in the Blood (animation) Annenberg Learner Carbonic Acid Buffer System In Human Blood Learn how carbonic acid and bicarbonate anion form a buffer system in human blood to maintain ph between 7.35 and 7.45. In this system, gaseous metabolic waste carbon dioxide reacts. Carbonic anhydrase is in red blood cells, renal tubules, gastric mucosa, and pancreatic cells. Learn how the bicarbonate buffer system, respiration and kidneys regulate blood ph within a narrow range. Carbonic Acid Buffer System In Human Blood.
From resources.wfsahq.org
Respiratory Physiology Part 2 WFSA Resources Carbonic Acid Buffer System In Human Blood Learn how carbonic acid and bicarbonate anion form a buffer system in human blood to maintain ph between 7.35 and 7.45. Other buffer systems in the human body include the phosphate buffer. Learn how the bicarbonate buffer system maintains ph in the blood and duodenum by balancing carbonic acid, bicarbonate ion, and. Learn how the bicarbonate buffer system, respiration and. Carbonic Acid Buffer System In Human Blood.
From www.paediatricsandchildhealthjournal.co.uk
Understanding blood gases/acidbase balance Paediatrics and Child Health Carbonic Acid Buffer System In Human Blood Learn how carbonic acid and bicarbonate anion form a buffer system in human blood to maintain ph between 7.35 and 7.45. Carbonic anhydrase is in red blood cells, renal tubules, gastric mucosa, and pancreatic cells. Other buffer systems in the human body include the phosphate buffer. The bicarbonate buffer system is the largest. Learn how the bicarbonate buffer system, respiration. Carbonic Acid Buffer System In Human Blood.
From www.slideserve.com
PPT Blood Buffers PowerPoint Presentation, free download ID2730981 Carbonic Acid Buffer System In Human Blood The bicarbonate buffer system is the largest. Find out how the respiratory and. Carbonic anhydrase is in red blood cells, renal tubules, gastric mucosa, and pancreatic cells. In this system, gaseous metabolic waste carbon dioxide reacts. Learn how the bicarbonate buffer system maintains ph in the blood and duodenum by balancing carbonic acid, bicarbonate ion, and. Other buffer systems in. Carbonic Acid Buffer System In Human Blood.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID Carbonic Acid Buffer System In Human Blood Carbonic anhydrase is in red blood cells, renal tubules, gastric mucosa, and pancreatic cells. Find out how the respiratory and. The bicarbonate buffer system is the largest. Learn how carbonic acid and bicarbonate anion form a buffer system in human blood to maintain ph between 7.35 and 7.45. Other buffer systems in the human body include the phosphate buffer. In. Carbonic Acid Buffer System In Human Blood.
From regulationlatest.blogspot.com
Blood Ph Regulation Homeostasis Carbonic Acid Buffer System In Human Blood Other buffer systems in the human body include the phosphate buffer. Learn how carbonic acid and bicarbonate anion form a buffer system in human blood to maintain ph between 7.35 and 7.45. Find out how the respiratory and. The bicarbonate buffer system is the largest. Learn how the bicarbonate buffer system maintains ph in the blood and duodenum by balancing. Carbonic Acid Buffer System In Human Blood.
From www.chegg.com
Solved The Bicarbonate Buffering System Increase H Carbonic Acid Buffer System In Human Blood In this system, gaseous metabolic waste carbon dioxide reacts. Other buffer systems in the human body include the phosphate buffer. Learn how the bicarbonate buffer system, respiration and kidneys regulate blood ph within a narrow range of 7.35 to 7.45. Learn how carbonic acid and bicarbonate anion form a buffer system in human blood to maintain ph between 7.35 and. Carbonic Acid Buffer System In Human Blood.
From simplemed.co.uk
5. Carbon Dioxide Transport SimpleMed Learning Medicine, Simplified Carbonic Acid Buffer System In Human Blood Other buffer systems in the human body include the phosphate buffer. Find out how the respiratory and. Carbonic anhydrase is in red blood cells, renal tubules, gastric mucosa, and pancreatic cells. Learn how the bicarbonate buffer system maintains ph in the blood and duodenum by balancing carbonic acid, bicarbonate ion, and. Learn how the bicarbonate buffer system, respiration and kidneys. Carbonic Acid Buffer System In Human Blood.
From www.youtube.com
Buffer action in the blood YouTube Carbonic Acid Buffer System In Human Blood Carbonic anhydrase is in red blood cells, renal tubules, gastric mucosa, and pancreatic cells. Learn how the bicarbonate buffer system, respiration and kidneys regulate blood ph within a narrow range of 7.35 to 7.45. Other buffer systems in the human body include the phosphate buffer. Learn how carbonic acid and bicarbonate anion form a buffer system in human blood to. Carbonic Acid Buffer System In Human Blood.
From books.byui.edu
Oxygen and Carbon Dioxide Transport in the Blood Carbonic Acid Buffer System In Human Blood Find out how the respiratory and. In this system, gaseous metabolic waste carbon dioxide reacts. Learn how the bicarbonate buffer system maintains ph in the blood and duodenum by balancing carbonic acid, bicarbonate ion, and. Learn how the bicarbonate buffer system, respiration and kidneys regulate blood ph within a narrow range of 7.35 to 7.45. Learn how carbonic acid and. Carbonic Acid Buffer System In Human Blood.
From barcelonageeks.com
Transporte de dirust de carbono en la sangre Barcelona Geeks Carbonic Acid Buffer System In Human Blood Learn how carbonic acid and bicarbonate anion form a buffer system in human blood to maintain ph between 7.35 and 7.45. The bicarbonate buffer system is the largest. Carbonic anhydrase is in red blood cells, renal tubules, gastric mucosa, and pancreatic cells. Learn how the bicarbonate buffer system maintains ph in the blood and duodenum by balancing carbonic acid, bicarbonate. Carbonic Acid Buffer System In Human Blood.
From www.slideshare.net
Buffer system Carbonic Acid Buffer System In Human Blood The bicarbonate buffer system is the largest. Learn how the bicarbonate buffer system, respiration and kidneys regulate blood ph within a narrow range of 7.35 to 7.45. In this system, gaseous metabolic waste carbon dioxide reacts. Learn how carbonic acid and bicarbonate anion form a buffer system in human blood to maintain ph between 7.35 and 7.45. Find out how. Carbonic Acid Buffer System In Human Blood.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Carbonic Acid Buffer System In Human Blood The bicarbonate buffer system is the largest. In this system, gaseous metabolic waste carbon dioxide reacts. Learn how carbonic acid and bicarbonate anion form a buffer system in human blood to maintain ph between 7.35 and 7.45. Find out how the respiratory and. Learn how the bicarbonate buffer system maintains ph in the blood and duodenum by balancing carbonic acid,. Carbonic Acid Buffer System In Human Blood.
From www.slideserve.com
PPT Chapter 27, part 2 PowerPoint Presentation, free download ID Carbonic Acid Buffer System In Human Blood In this system, gaseous metabolic waste carbon dioxide reacts. Find out how the respiratory and. Learn how the bicarbonate buffer system, respiration and kidneys regulate blood ph within a narrow range of 7.35 to 7.45. Other buffer systems in the human body include the phosphate buffer. The bicarbonate buffer system is the largest. Learn how carbonic acid and bicarbonate anion. Carbonic Acid Buffer System In Human Blood.
From www.slideserve.com
PPT ARTERIAL BLOOD GAS ANALYSIS PowerPoint Presentation ID5086981 Carbonic Acid Buffer System In Human Blood Learn how the bicarbonate buffer system maintains ph in the blood and duodenum by balancing carbonic acid, bicarbonate ion, and. Find out how the respiratory and. Other buffer systems in the human body include the phosphate buffer. Learn how the bicarbonate buffer system, respiration and kidneys regulate blood ph within a narrow range of 7.35 to 7.45. The bicarbonate buffer. Carbonic Acid Buffer System In Human Blood.
From www.slideserve.com
PPT Maintaining Blood pH PowerPoint Presentation, free download ID Carbonic Acid Buffer System In Human Blood Find out how the respiratory and. Learn how the bicarbonate buffer system, respiration and kidneys regulate blood ph within a narrow range of 7.35 to 7.45. Other buffer systems in the human body include the phosphate buffer. The bicarbonate buffer system is the largest. Learn how the bicarbonate buffer system maintains ph in the blood and duodenum by balancing carbonic. Carbonic Acid Buffer System In Human Blood.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Carbonic Acid Buffer System In Human Blood Carbonic anhydrase is in red blood cells, renal tubules, gastric mucosa, and pancreatic cells. Find out how the respiratory and. Learn how the bicarbonate buffer system maintains ph in the blood and duodenum by balancing carbonic acid, bicarbonate ion, and. Other buffer systems in the human body include the phosphate buffer. Learn how carbonic acid and bicarbonate anion form a. Carbonic Acid Buffer System In Human Blood.
From quizlet.com
Bicarbonate Buffer system Diagram Quizlet Carbonic Acid Buffer System In Human Blood The bicarbonate buffer system is the largest. Find out how the respiratory and. Other buffer systems in the human body include the phosphate buffer. Learn how the bicarbonate buffer system maintains ph in the blood and duodenum by balancing carbonic acid, bicarbonate ion, and. Learn how the bicarbonate buffer system, respiration and kidneys regulate blood ph within a narrow range. Carbonic Acid Buffer System In Human Blood.
From www.slideserve.com
PPT RESPIRATORY SYSTEM 3 PowerPoint Presentation, free download Carbonic Acid Buffer System In Human Blood In this system, gaseous metabolic waste carbon dioxide reacts. The bicarbonate buffer system is the largest. Carbonic anhydrase is in red blood cells, renal tubules, gastric mucosa, and pancreatic cells. Learn how carbonic acid and bicarbonate anion form a buffer system in human blood to maintain ph between 7.35 and 7.45. Other buffer systems in the human body include the. Carbonic Acid Buffer System In Human Blood.
From labpedia.net
Acidbase Balance Part 3 Respiratory Acidosis and Respiratory Carbonic Acid Buffer System In Human Blood Other buffer systems in the human body include the phosphate buffer. In this system, gaseous metabolic waste carbon dioxide reacts. Learn how the bicarbonate buffer system maintains ph in the blood and duodenum by balancing carbonic acid, bicarbonate ion, and. Carbonic anhydrase is in red blood cells, renal tubules, gastric mucosa, and pancreatic cells. Learn how the bicarbonate buffer system,. Carbonic Acid Buffer System In Human Blood.
From www.pinterest.ca
bicarbonate buffer system, example of multiple equilibria Teaching Carbonic Acid Buffer System In Human Blood Carbonic anhydrase is in red blood cells, renal tubules, gastric mucosa, and pancreatic cells. In this system, gaseous metabolic waste carbon dioxide reacts. Learn how the bicarbonate buffer system, respiration and kidneys regulate blood ph within a narrow range of 7.35 to 7.45. Find out how the respiratory and. The bicarbonate buffer system is the largest. Learn how the bicarbonate. Carbonic Acid Buffer System In Human Blood.
From fieselina.blogspot.com
Carbonate Buffer System Explained carbonate Carbonic Acid Buffer System In Human Blood Learn how the bicarbonate buffer system, respiration and kidneys regulate blood ph within a narrow range of 7.35 to 7.45. Find out how the respiratory and. Carbonic anhydrase is in red blood cells, renal tubules, gastric mucosa, and pancreatic cells. Learn how carbonic acid and bicarbonate anion form a buffer system in human blood to maintain ph between 7.35 and. Carbonic Acid Buffer System In Human Blood.