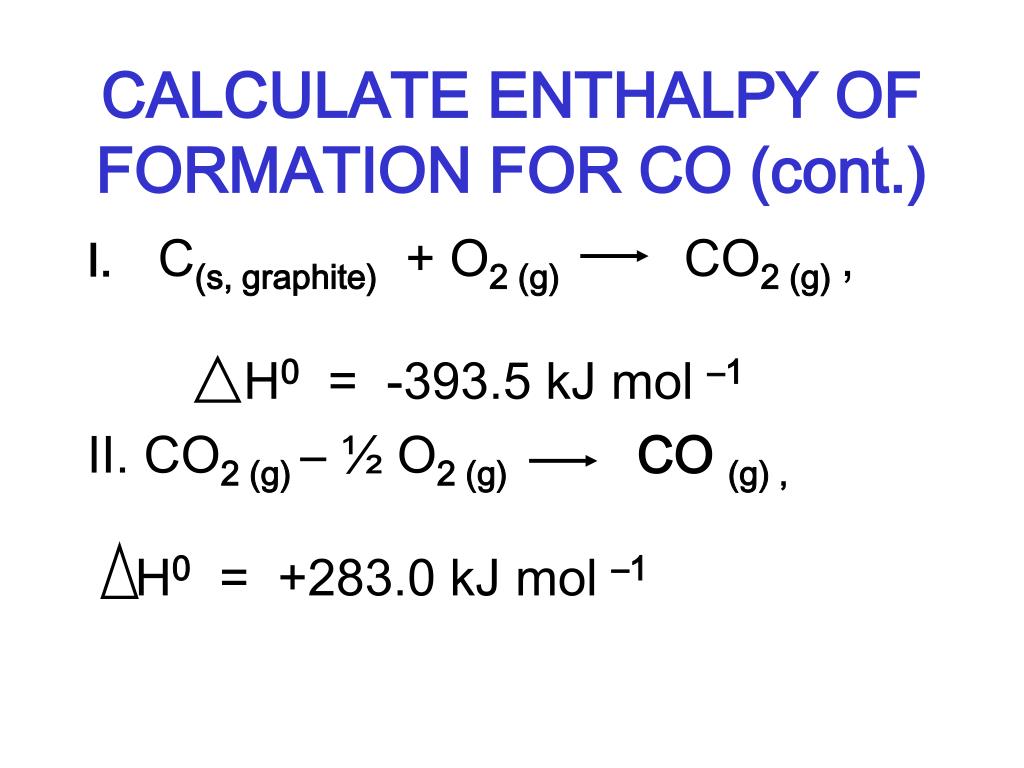
Standard Enthalpy Of Reaction Vs Formation . The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. The enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy of reaction is the. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas,. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.
from www.slideserve.com
The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas,. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy of reaction is the. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard.
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation
Standard Enthalpy Of Reaction Vs Formation The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas,. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas,. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy of reaction is the. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when.
From www.slideserve.com
PPT Chapter 15 Standard enthalpy change of a reaction PowerPoint Standard Enthalpy Of Reaction Vs Formation Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas,. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole. Standard Enthalpy Of Reaction Vs Formation.
From www.youtube.com
How to Calculate the Standard Enthalpy of a Reaction from the Standard Standard Enthalpy Of Reaction Vs Formation A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows in chemistry and thermodynamics,. Standard Enthalpy Of Reaction Vs Formation.
From www.writework.com
A comparison between the Enthalpy of formation of MgO acquired via a Standard Enthalpy Of Reaction Vs Formation A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy of reaction is the.. Standard Enthalpy Of Reaction Vs Formation.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Reaction Vs Formation Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. The enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy of reaction is the. The magnitude of δ h for a reaction depends on the physical states of the reactants. Standard Enthalpy Of Reaction Vs Formation.
From pediaa.com
What is the Difference Between Enthalpy of Formation and Enthalpy of Standard Enthalpy Of Reaction Vs Formation The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas,. The enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy of reaction is the. A standard enthalpy of formation δ h f ° δ h f °. Standard Enthalpy Of Reaction Vs Formation.
From www.nagwa.com
Question Video Using Standard Enthalpies of Formation to Find Δ퐻⦵ in Standard Enthalpy Of Reaction Vs Formation The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. The enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard. Standard Enthalpy Of Reaction Vs Formation.
From pediaa.com
Difference Between Heat of Formation and Heat of Reaction Definition Standard Enthalpy Of Reaction Vs Formation A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The magnitude of δ h for a reaction depends on the physical states of the. Standard Enthalpy Of Reaction Vs Formation.
From www.vrogue.co
Standard Enthalpy Of Reaction Units Slide Share vrogue.co Standard Enthalpy Of Reaction Vs Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements. Standard Enthalpy Of Reaction Vs Formation.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Reaction Vs Formation A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas,. The enthalpy of formation is the enthalpy. Standard Enthalpy Of Reaction Vs Formation.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Reaction Vs Formation The enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy of reaction is the. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy. Standard Enthalpy Of Reaction Vs Formation.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Reaction Vs Formation The enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy of reaction is the. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the. Standard Enthalpy Of Reaction Vs Formation.
From www.youtube.com
Std Heat of Formation versus Bond Energy YouTube Standard Enthalpy Of Reaction Vs Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The enthalpy of formation. Standard Enthalpy Of Reaction Vs Formation.
From scienceinfo.com
Enthalpy Introduction, Calculation, Enthalpy change, Importance Standard Enthalpy Of Reaction Vs Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction. Standard Enthalpy Of Reaction Vs Formation.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Standard Enthalpy Of Reaction Vs Formation The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in. Standard Enthalpy Of Reaction Vs Formation.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Reaction Vs Formation Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements. Standard Enthalpy Of Reaction Vs Formation.
From www.numerade.com
Consider the following reaction 2 H2S (g) + 3 O2 (g) → 2 SO2 (g) + 2 Standard Enthalpy Of Reaction Vs Formation Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. The enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy of reaction is the. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change. Standard Enthalpy Of Reaction Vs Formation.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Reaction Vs Formation The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas,. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a. Standard Enthalpy Of Reaction Vs Formation.
From slideplayer.com
General Chemistry CHEM 101 (3+1+0). ppt download Standard Enthalpy Of Reaction Vs Formation The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. A standard enthalpy of formation δ h f ° δ h f ° is an. Standard Enthalpy Of Reaction Vs Formation.
From www.slideserve.com
PPT THERMOCHEMISTRY PowerPoint Presentation, free download ID5072129 Standard Enthalpy Of Reaction Vs Formation The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas,. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard. Standard Enthalpy Of Reaction Vs Formation.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Reaction Vs Formation Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas,. The enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy. Standard Enthalpy Of Reaction Vs Formation.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Enthalpy Of Reaction Vs Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy of reaction is the. The standard enthalpy of formation is a measure of the. Standard Enthalpy Of Reaction Vs Formation.
From chem.libretexts.org
5.7 Enthalpies of Formation Chemistry LibreTexts Standard Enthalpy Of Reaction Vs Formation The enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy of reaction is the. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. The standard enthalpy of formation is a measure of the energy released or consumed when one. Standard Enthalpy Of Reaction Vs Formation.
From www.slideserve.com
PPT Enthalpies of Formation and Reaction PowerPoint Presentation Standard Enthalpy Of Reaction Vs Formation A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change. Standard Enthalpy Of Reaction Vs Formation.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Reaction Vs Formation A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpy of formation is the. Standard Enthalpy Of Reaction Vs Formation.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Reaction Vs Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. The magnitude of δ h for a reaction depends on the physical states of the. Standard Enthalpy Of Reaction Vs Formation.
From www.youtube.com
5.1 Standard enthalpy changes of formation and combustion YouTube Standard Enthalpy Of Reaction Vs Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. The enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state,. Standard Enthalpy Of Reaction Vs Formation.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Reaction Vs Formation The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas,. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard. Standard Enthalpy Of Reaction Vs Formation.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Reaction Vs Formation The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas,. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent. Standard Enthalpy Of Reaction Vs Formation.
From www.youtube.com
Writing Equations for Standard Enthalpy of Formation Examples YouTube Standard Enthalpy Of Reaction Vs Formation A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy of reaction is the.. Standard Enthalpy Of Reaction Vs Formation.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Reaction Vs Formation A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas,. The standard enthalpy of formation is a. Standard Enthalpy Of Reaction Vs Formation.
From chemistry.stackexchange.com
thermodynamics Calculating Enthalpy of formation versus Calculating Standard Enthalpy Of Reaction Vs Formation The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas,. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The enthalpy. Standard Enthalpy Of Reaction Vs Formation.
From mavink.com
Enthalpy Of Formation Equation Standard Enthalpy Of Reaction Vs Formation The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas,. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. The enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy. Standard Enthalpy Of Reaction Vs Formation.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Enthalpy Of Reaction Vs Formation Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound. Standard Enthalpy Of Reaction Vs Formation.
From www.msjchem.com
Topic 5 Energetics MSJChem Tutorial videos for IB Chemistry Standard Enthalpy Of Reaction Vs Formation The enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy of reaction is the. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. A standard enthalpy of formation δ h f ° δ h. Standard Enthalpy Of Reaction Vs Formation.
From www.youtube.com
6.6 Standard Enthalpy of Formation and Reaction YouTube Standard Enthalpy Of Reaction Vs Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy of formation is the enthalpy change when one mole of any. Standard Enthalpy Of Reaction Vs Formation.