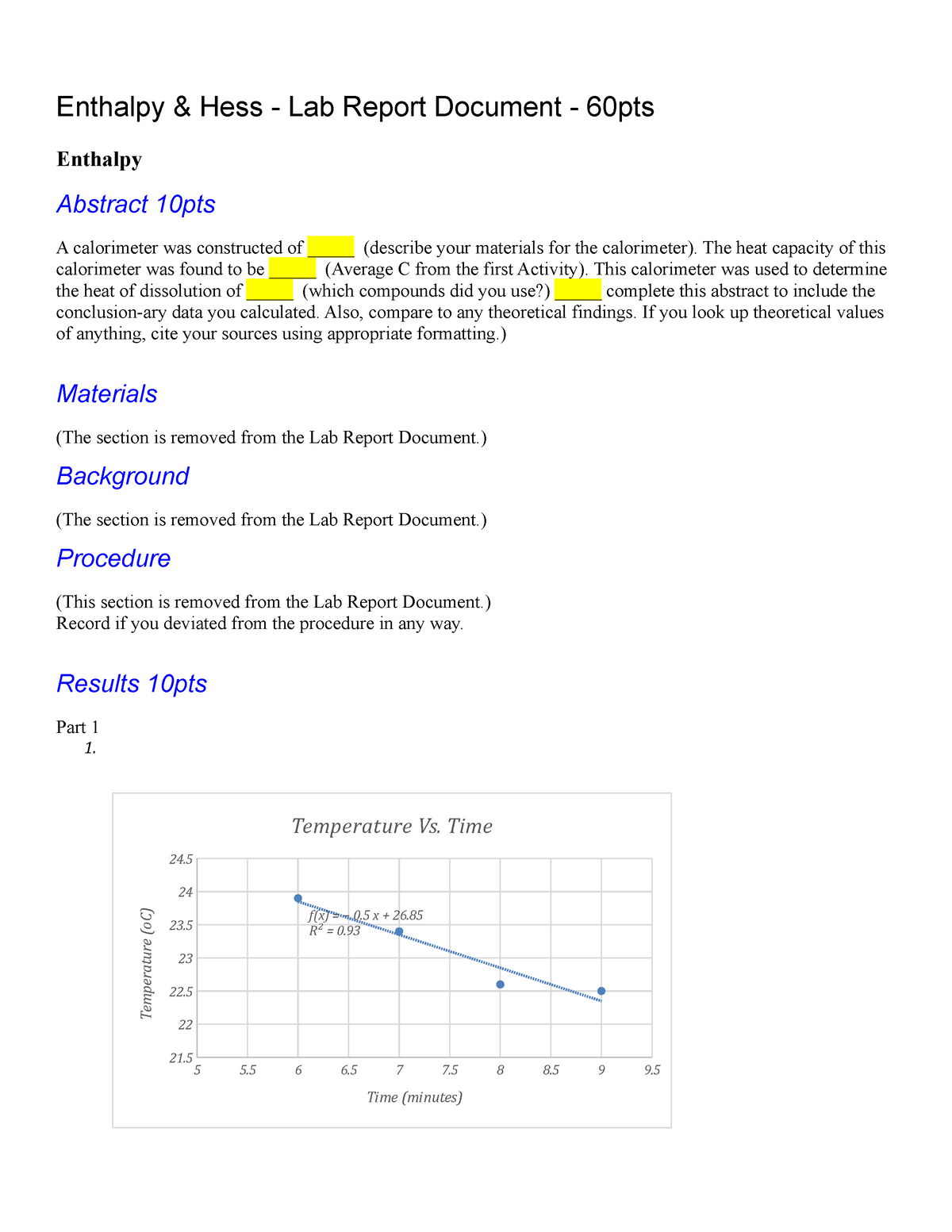
Lab Enthalpy Assignment Reflect On The Lab . The material with the greatest specific heat. Determining molar enthalpy of neutralization. Mark baillie coordinated the modifications of this activity for implementation in a 15 week fall course, with the help of elena lisitsyna and. Y = 0 + 21. To use mass and temperature data to do computations involving. Your experimental value was likely different. The purpose of this experiment is to determine enthalpy changes (δhrxn) of five. Water is also capable of retaining heat, this enables other liquids like the naoh and hcl to absorb the heat. It is the sum of internal energy and product of pressure and volume. Determining the enthalpy of a chemical reaction. Chemistry 2003 bmwf professor olga lavinda. 1) enthalpy is a thermodynamic entity measuring energy of a system. Study with quizlet and memorize flashcards containing terms like to do this computation for each of. 0 5 10 15 20 25 30. What were the learning goals of this lab experiment?
from www.studocu.com
Y = 0 + 21. Study with quizlet and memorize flashcards containing terms like to do this computation for each of. To use mass and temperature data to do computations involving. It is the sum of internal energy and product of pressure and volume. Water is also capable of retaining heat, this enables other liquids like the naoh and hcl to absorb the heat. Your experimental value was likely different. The purpose of this experiment is to determine enthalpy changes (δhrxn) of five. Chemistry 2003 bmwf professor olga lavinda. 0 5 10 15 20 25 30. Determining the enthalpy of a chemical reaction.
Lab Report Document Enthalpy and Hess s Law Enthalpy & Hess Lab
Lab Enthalpy Assignment Reflect On The Lab To use mass and temperature data to do computations involving. Chemistry 2003 bmwf professor olga lavinda. 1) enthalpy is a thermodynamic entity measuring energy of a system. What were the learning goals of this lab experiment? Determining the enthalpy of a chemical reaction. Study with quizlet and memorize flashcards containing terms like to do this computation for each of. The material with the greatest specific heat. Your experimental value was likely different. Determining molar enthalpy of neutralization. 0 5 10 15 20 25 30. It is the sum of internal energy and product of pressure and volume. Mark baillie coordinated the modifications of this activity for implementation in a 15 week fall course, with the help of elena lisitsyna and. Y = 0 + 21. To use mass and temperature data to do computations involving. Water is also capable of retaining heat, this enables other liquids like the naoh and hcl to absorb the heat. The purpose of this experiment is to determine enthalpy changes (δhrxn) of five.
From www.studypool.com
SOLUTION Scientific Method Post Lab Assignment Studypool Lab Enthalpy Assignment Reflect On The Lab 0 5 10 15 20 25 30. The purpose of this experiment is to determine enthalpy changes (δhrxn) of five. The material with the greatest specific heat. 1) enthalpy is a thermodynamic entity measuring energy of a system. What were the learning goals of this lab experiment? Determining the enthalpy of a chemical reaction. It is the sum of internal. Lab Enthalpy Assignment Reflect On The Lab.
From www.studocu.com
Lab Report on the Molar Enthalpy Change of Combustion An experiment Lab Enthalpy Assignment Reflect On The Lab Your experimental value was likely different. Determining molar enthalpy of neutralization. The material with the greatest specific heat. Chemistry 2003 bmwf professor olga lavinda. The purpose of this experiment is to determine enthalpy changes (δhrxn) of five. Determining the enthalpy of a chemical reaction. Mark baillie coordinated the modifications of this activity for implementation in a 15 week fall course,. Lab Enthalpy Assignment Reflect On The Lab.
From phdessay.com
Enthalpy Lab Background Assignment Example Lab Enthalpy Assignment Reflect On The Lab Your experimental value was likely different. Water is also capable of retaining heat, this enables other liquids like the naoh and hcl to absorb the heat. The purpose of this experiment is to determine enthalpy changes (δhrxn) of five. What were the learning goals of this lab experiment? 0 5 10 15 20 25 30. Determining molar enthalpy of neutralization.. Lab Enthalpy Assignment Reflect On The Lab.
From www.coursehero.com
Laboratory Assignment Molar Enthalpy of Neutralization LABORATORY Lab Enthalpy Assignment Reflect On The Lab It is the sum of internal energy and product of pressure and volume. What were the learning goals of this lab experiment? Your experimental value was likely different. 0 5 10 15 20 25 30. Mark baillie coordinated the modifications of this activity for implementation in a 15 week fall course, with the help of elena lisitsyna and. 1) enthalpy. Lab Enthalpy Assignment Reflect On The Lab.
From www.chegg.com
Solved Prelab Assignment Enthalpy of Reaction Review the Lab Enthalpy Assignment Reflect On The Lab Determining the enthalpy of a chemical reaction. Your experimental value was likely different. Chemistry 2003 bmwf professor olga lavinda. What were the learning goals of this lab experiment? The purpose of this experiment is to determine enthalpy changes (δhrxn) of five. Determining molar enthalpy of neutralization. Study with quizlet and memorize flashcards containing terms like to do this computation for. Lab Enthalpy Assignment Reflect On The Lab.
From www.studypool.com
SOLUTION C Enthalpy Change Lab Assignment Studypool Lab Enthalpy Assignment Reflect On The Lab 0 5 10 15 20 25 30. Determining the enthalpy of a chemical reaction. To use mass and temperature data to do computations involving. The material with the greatest specific heat. Water is also capable of retaining heat, this enables other liquids like the naoh and hcl to absorb the heat. What were the learning goals of this lab experiment?. Lab Enthalpy Assignment Reflect On The Lab.
From www.studocu.com
Enthalpy Lab Report idkkkk Enthalpy Lab Report Kayla Shedd Lab Enthalpy Assignment Reflect On The Lab What were the learning goals of this lab experiment? Determining the enthalpy of a chemical reaction. To use mass and temperature data to do computations involving. Water is also capable of retaining heat, this enables other liquids like the naoh and hcl to absorb the heat. 0 5 10 15 20 25 30. Mark baillie coordinated the modifications of this. Lab Enthalpy Assignment Reflect On The Lab.
From www.thinkswap.com
Chemistry enthalpy report Chemistry Year 11 SACE Thinkswap Lab Enthalpy Assignment Reflect On The Lab Water is also capable of retaining heat, this enables other liquids like the naoh and hcl to absorb the heat. Mark baillie coordinated the modifications of this activity for implementation in a 15 week fall course, with the help of elena lisitsyna and. Determining molar enthalpy of neutralization. The material with the greatest specific heat. The purpose of this experiment. Lab Enthalpy Assignment Reflect On The Lab.
From cupsoguepictures.com
😎 Enthalpy of reaction lab. Enthalpy Lab Report Essay. 20190222 Lab Enthalpy Assignment Reflect On The Lab Mark baillie coordinated the modifications of this activity for implementation in a 15 week fall course, with the help of elena lisitsyna and. Your experimental value was likely different. Study with quizlet and memorize flashcards containing terms like to do this computation for each of. The purpose of this experiment is to determine enthalpy changes (δhrxn) of five. Determining the. Lab Enthalpy Assignment Reflect On The Lab.
From studylib.net
Enthalpy Lab Lab Enthalpy Assignment Reflect On The Lab Your experimental value was likely different. It is the sum of internal energy and product of pressure and volume. Water is also capable of retaining heat, this enables other liquids like the naoh and hcl to absorb the heat. Determining molar enthalpy of neutralization. Study with quizlet and memorize flashcards containing terms like to do this computation for each of.. Lab Enthalpy Assignment Reflect On The Lab.
From studylib.net
LABORATORY EXERCISE 8 Enthalpy Change (Hess` Law) Lab Enthalpy Assignment Reflect On The Lab What were the learning goals of this lab experiment? 1) enthalpy is a thermodynamic entity measuring energy of a system. Your experimental value was likely different. To use mass and temperature data to do computations involving. The material with the greatest specific heat. Mark baillie coordinated the modifications of this activity for implementation in a 15 week fall course, with. Lab Enthalpy Assignment Reflect On The Lab.
From www.studocu.com
Lab 11 Pre Lab Prelab for 11 Lab 11 Enthalpy of Formation PreLab Lab Enthalpy Assignment Reflect On The Lab What were the learning goals of this lab experiment? The material with the greatest specific heat. It is the sum of internal energy and product of pressure and volume. Y = 0 + 21. Study with quizlet and memorize flashcards containing terms like to do this computation for each of. 1) enthalpy is a thermodynamic entity measuring energy of a. Lab Enthalpy Assignment Reflect On The Lab.
From www.academia.edu
(DOC) Enthalpy of Neutralization Procedure/Experiment Jonniel Vince Lab Enthalpy Assignment Reflect On The Lab Determining molar enthalpy of neutralization. Chemistry 2003 bmwf professor olga lavinda. The purpose of this experiment is to determine enthalpy changes (δhrxn) of five. Study with quizlet and memorize flashcards containing terms like to do this computation for each of. Mark baillie coordinated the modifications of this activity for implementation in a 15 week fall course, with the help of. Lab Enthalpy Assignment Reflect On The Lab.
From www.studocu.com
Lab 3 Enthalpy Change F22 Lab 3 Assignment Enthalpy Change of a Lab Enthalpy Assignment Reflect On The Lab Your experimental value was likely different. Mark baillie coordinated the modifications of this activity for implementation in a 15 week fall course, with the help of elena lisitsyna and. It is the sum of internal energy and product of pressure and volume. Y = 0 + 21. Water is also capable of retaining heat, this enables other liquids like the. Lab Enthalpy Assignment Reflect On The Lab.
From www.studypool.com
SOLUTION Chemistry b unit 2 lab enthalpy student guide Studypool Lab Enthalpy Assignment Reflect On The Lab 1) enthalpy is a thermodynamic entity measuring energy of a system. Chemistry 2003 bmwf professor olga lavinda. Determining molar enthalpy of neutralization. Your experimental value was likely different. The purpose of this experiment is to determine enthalpy changes (δhrxn) of five. To use mass and temperature data to do computations involving. What were the learning goals of this lab experiment?. Lab Enthalpy Assignment Reflect On The Lab.
From www.chegg.com
Part 5 Measuring the enthalpy of neutralization Lab Enthalpy Assignment Reflect On The Lab Y = 0 + 21. Water is also capable of retaining heat, this enables other liquids like the naoh and hcl to absorb the heat. The material with the greatest specific heat. 0 5 10 15 20 25 30. Determining the enthalpy of a chemical reaction. It is the sum of internal energy and product of pressure and volume. What. Lab Enthalpy Assignment Reflect On The Lab.
From www.studocu.com
Pre lab 3 pre lab report on identifying enthalpy of reaction using Lab Enthalpy Assignment Reflect On The Lab Study with quizlet and memorize flashcards containing terms like to do this computation for each of. Determining the enthalpy of a chemical reaction. Water is also capable of retaining heat, this enables other liquids like the naoh and hcl to absorb the heat. Your experimental value was likely different. Mark baillie coordinated the modifications of this activity for implementation in. Lab Enthalpy Assignment Reflect On The Lab.
From www.studocu.com
Lab Report Document Enthalpy and Hess s Law Enthalpy & Hess Lab Lab Enthalpy Assignment Reflect On The Lab Study with quizlet and memorize flashcards containing terms like to do this computation for each of. Mark baillie coordinated the modifications of this activity for implementation in a 15 week fall course, with the help of elena lisitsyna and. The purpose of this experiment is to determine enthalpy changes (δhrxn) of five. Determining the enthalpy of a chemical reaction. The. Lab Enthalpy Assignment Reflect On The Lab.
From www.youtube.com
Enthalpy of Combustion Lab Explained YouTube Lab Enthalpy Assignment Reflect On The Lab What were the learning goals of this lab experiment? Water is also capable of retaining heat, this enables other liquids like the naoh and hcl to absorb the heat. The material with the greatest specific heat. Chemistry 2003 bmwf professor olga lavinda. It is the sum of internal energy and product of pressure and volume. Mark baillie coordinated the modifications. Lab Enthalpy Assignment Reflect On The Lab.
From brainly.com
Lab report enthalpy If you have the actual lab report pls link it but Lab Enthalpy Assignment Reflect On The Lab It is the sum of internal energy and product of pressure and volume. Chemistry 2003 bmwf professor olga lavinda. 0 5 10 15 20 25 30. Water is also capable of retaining heat, this enables other liquids like the naoh and hcl to absorb the heat. 1) enthalpy is a thermodynamic entity measuring energy of a system. What were the. Lab Enthalpy Assignment Reflect On The Lab.
From www.studocu.com
Lab Report Enthalpy Lab Enthalpy Lab Purpose In this inquiry lab, I Lab Enthalpy Assignment Reflect On The Lab Your experimental value was likely different. Determining molar enthalpy of neutralization. To use mass and temperature data to do computations involving. Determining the enthalpy of a chemical reaction. Mark baillie coordinated the modifications of this activity for implementation in a 15 week fall course, with the help of elena lisitsyna and. Chemistry 2003 bmwf professor olga lavinda. 0 5 10. Lab Enthalpy Assignment Reflect On The Lab.
From www.studocu.com
2b.Lab Assignment Enthalpy of Salts Lab Assignment Enthalpy of Salts Lab Enthalpy Assignment Reflect On The Lab 1) enthalpy is a thermodynamic entity measuring energy of a system. The material with the greatest specific heat. Determining the enthalpy of a chemical reaction. Y = 0 + 21. Study with quizlet and memorize flashcards containing terms like to do this computation for each of. Determining molar enthalpy of neutralization. Water is also capable of retaining heat, this enables. Lab Enthalpy Assignment Reflect On The Lab.
From studylib.net
Experiment 9 Enthalpy of Formation of Magnesium Oxide Lab Enthalpy Assignment Reflect On The Lab Your experimental value was likely different. Water is also capable of retaining heat, this enables other liquids like the naoh and hcl to absorb the heat. Study with quizlet and memorize flashcards containing terms like to do this computation for each of. Chemistry 2003 bmwf professor olga lavinda. The material with the greatest specific heat. The purpose of this experiment. Lab Enthalpy Assignment Reflect On The Lab.
From www.vernier.com
Determining the Enthalpy of a Chemical Reaction > Experiment 13 from Lab Enthalpy Assignment Reflect On The Lab Your experimental value was likely different. What were the learning goals of this lab experiment? Determining the enthalpy of a chemical reaction. Chemistry 2003 bmwf professor olga lavinda. 0 5 10 15 20 25 30. Water is also capable of retaining heat, this enables other liquids like the naoh and hcl to absorb the heat. Mark baillie coordinated the modifications. Lab Enthalpy Assignment Reflect On The Lab.
From www.studypool.com
SOLUTION C Enthalpy Change Lab Assignment Studypool Lab Enthalpy Assignment Reflect On The Lab Determining the enthalpy of a chemical reaction. The purpose of this experiment is to determine enthalpy changes (δhrxn) of five. Your experimental value was likely different. 1) enthalpy is a thermodynamic entity measuring energy of a system. To use mass and temperature data to do computations involving. Study with quizlet and memorize flashcards containing terms like to do this computation. Lab Enthalpy Assignment Reflect On The Lab.
From www.answersarena.com
[Solved] Using the general properties of reaction enthalpy. Lab Enthalpy Assignment Reflect On The Lab Your experimental value was likely different. Chemistry 2003 bmwf professor olga lavinda. Determining the enthalpy of a chemical reaction. Mark baillie coordinated the modifications of this activity for implementation in a 15 week fall course, with the help of elena lisitsyna and. Water is also capable of retaining heat, this enables other liquids like the naoh and hcl to absorb. Lab Enthalpy Assignment Reflect On The Lab.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Lab Enthalpy Assignment Reflect On The Lab Mark baillie coordinated the modifications of this activity for implementation in a 15 week fall course, with the help of elena lisitsyna and. Study with quizlet and memorize flashcards containing terms like to do this computation for each of. Determining the enthalpy of a chemical reaction. 1) enthalpy is a thermodynamic entity measuring energy of a system. To use mass. Lab Enthalpy Assignment Reflect On The Lab.
From studylib.net
Enthalpy Lab Lab Enthalpy Assignment Reflect On The Lab Determining molar enthalpy of neutralization. What were the learning goals of this lab experiment? The purpose of this experiment is to determine enthalpy changes (δhrxn) of five. Chemistry 2003 bmwf professor olga lavinda. Y = 0 + 21. Mark baillie coordinated the modifications of this activity for implementation in a 15 week fall course, with the help of elena lisitsyna. Lab Enthalpy Assignment Reflect On The Lab.
From www.studocu.com
Lab Report 7 Goal of Experiment Enthalpy of reaction (Hrxn) is the Lab Enthalpy Assignment Reflect On The Lab To use mass and temperature data to do computations involving. 1) enthalpy is a thermodynamic entity measuring energy of a system. What were the learning goals of this lab experiment? 0 5 10 15 20 25 30. Mark baillie coordinated the modifications of this activity for implementation in a 15 week fall course, with the help of elena lisitsyna and.. Lab Enthalpy Assignment Reflect On The Lab.
From www.studocu.com
Lab Enthalpy Assignment Enthalpy Report Purpose Explore the Lab Enthalpy Assignment Reflect On The Lab Chemistry 2003 bmwf professor olga lavinda. Mark baillie coordinated the modifications of this activity for implementation in a 15 week fall course, with the help of elena lisitsyna and. To use mass and temperature data to do computations involving. Study with quizlet and memorize flashcards containing terms like to do this computation for each of. Determining the enthalpy of a. Lab Enthalpy Assignment Reflect On The Lab.
From www.numerade.com
SOLVED Experiment 3 PreLaboratory Assignment Enthalpy and Entropy Lab Enthalpy Assignment Reflect On The Lab Study with quizlet and memorize flashcards containing terms like to do this computation for each of. The purpose of this experiment is to determine enthalpy changes (δhrxn) of five. Your experimental value was likely different. To use mass and temperature data to do computations involving. 1) enthalpy is a thermodynamic entity measuring energy of a system. 0 5 10 15. Lab Enthalpy Assignment Reflect On The Lab.
From www.studypool.com
SOLUTION C Enthalpy Change Lab Assignment Studypool Lab Enthalpy Assignment Reflect On The Lab The purpose of this experiment is to determine enthalpy changes (δhrxn) of five. 1) enthalpy is a thermodynamic entity measuring energy of a system. The material with the greatest specific heat. Determining the enthalpy of a chemical reaction. Your experimental value was likely different. What were the learning goals of this lab experiment? Mark baillie coordinated the modifications of this. Lab Enthalpy Assignment Reflect On The Lab.
From www.studypool.com
SOLUTION Chemistry b unit 2 lab enthalpy student guide Studypool Lab Enthalpy Assignment Reflect On The Lab What were the learning goals of this lab experiment? It is the sum of internal energy and product of pressure and volume. The purpose of this experiment is to determine enthalpy changes (δhrxn) of five. 0 5 10 15 20 25 30. 1) enthalpy is a thermodynamic entity measuring energy of a system. Study with quizlet and memorize flashcards containing. Lab Enthalpy Assignment Reflect On The Lab.
From www.studocu.com
Enthalpy lab report Lab Chemistry 2003 BMWF Professor Olga Lavinda Lab Enthalpy Assignment Reflect On The Lab Mark baillie coordinated the modifications of this activity for implementation in a 15 week fall course, with the help of elena lisitsyna and. It is the sum of internal energy and product of pressure and volume. The purpose of this experiment is to determine enthalpy changes (δhrxn) of five. Study with quizlet and memorize flashcards containing terms like to do. Lab Enthalpy Assignment Reflect On The Lab.
From www.vernier.com
Enthalpy Changes > Experiment 9 from Investigating Chemistry through Lab Enthalpy Assignment Reflect On The Lab The material with the greatest specific heat. The purpose of this experiment is to determine enthalpy changes (δhrxn) of five. Mark baillie coordinated the modifications of this activity for implementation in a 15 week fall course, with the help of elena lisitsyna and. Your experimental value was likely different. It is the sum of internal energy and product of pressure. Lab Enthalpy Assignment Reflect On The Lab.