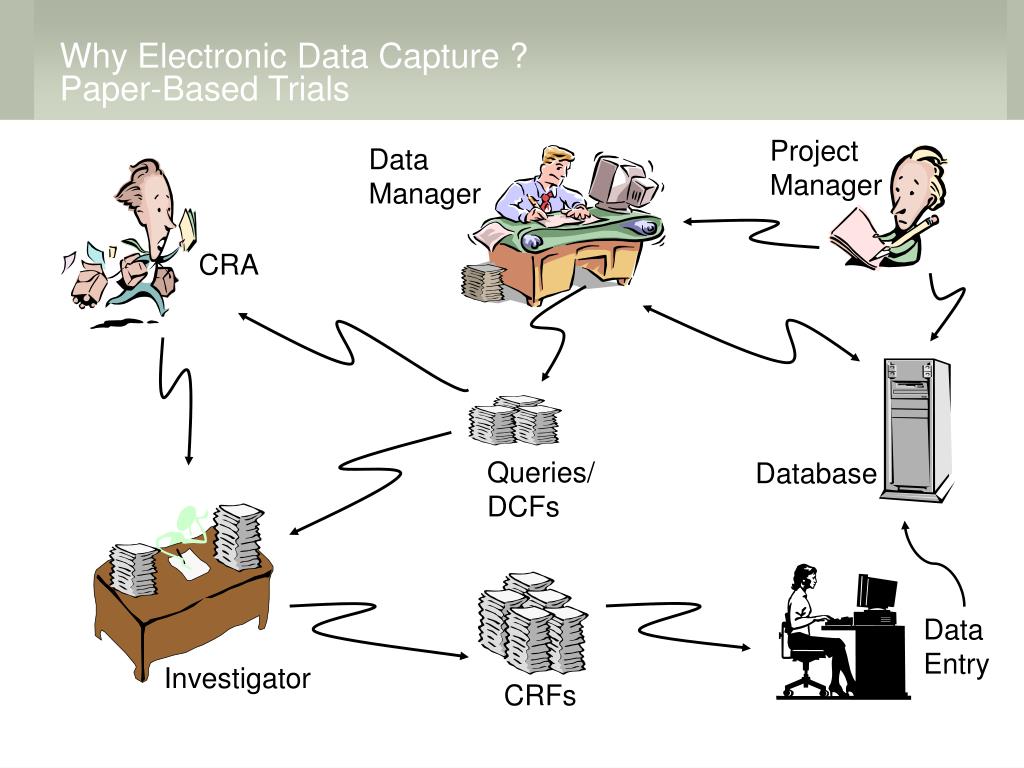
What Is Electronic Data Capture . what is edc in clinical trials? an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. Edc in clinical trials is a software system for collecting, managing, and storing data.
from www.slideserve.com
what is edc in clinical trials? an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. Edc in clinical trials is a software system for collecting, managing, and storing data. electronic data capture (edc) refers to the systematic process of collecting, managing and storing data.
PPT Breaking down the Barriers Electronic Data Capture PowerPoint
What Is Electronic Data Capture electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. Edc in clinical trials is a software system for collecting, managing, and storing data. what is edc in clinical trials?
From conductscience.com
Electronic Data Capture Conduct Science What Is Electronic Data Capture Edc in clinical trials is a software system for collecting, managing, and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. what is edc in clinical trials? electronic data capture (edc). What Is Electronic Data Capture.
From www.slideserve.com
PPT Breaking down the Barriers Electronic Data Capture PowerPoint What Is Electronic Data Capture electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. Edc in clinical trials is a software system for collecting, managing, and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage. What Is Electronic Data Capture.
From www.egnyte.com
Electronic Data Capture Fundamentals Egnyte What Is Electronic Data Capture electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. what is edc in clinical trials? Edc in clinical. What Is Electronic Data Capture.
From erpfm.com
What is a electronic data capture terminal? ERPfm What Is Electronic Data Capture Edc in clinical trials is a software system for collecting, managing, and storing data. electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage. What Is Electronic Data Capture.
From www.openclinica.com
The Fundamentals of Electronic Data Capture OpenClinica What Is Electronic Data Capture what is edc in clinical trials? an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. Edc in clinical trials is a software system for collecting, managing, and storing data. electronic data capture (edc). What Is Electronic Data Capture.
From www.clincapture.com
Captivate® Electronic Data Capture (EDC) ClinCapture What Is Electronic Data Capture an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. what is edc in clinical trials? Edc in clinical trials is a software system for collecting, managing, and storing data. electronic data capture (edc). What Is Electronic Data Capture.
From credevo.com
Electronic Data Capture (EDC) In Clinical Trials Credevo Articles What Is Electronic Data Capture electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. what is edc in clinical trials? an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. Edc in clinical. What Is Electronic Data Capture.
From clinicalpursuit.com
Benefits of Electronic Data Capture Systems What Is Electronic Data Capture an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. Edc in clinical trials is a software system for collecting,. What Is Electronic Data Capture.
From www.slideserve.com
PPT Using EDCRave to Conduct Clinical Trials at Genentech PowerPoint What Is Electronic Data Capture electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. what is edc in clinical trials? an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. Edc in clinical. What Is Electronic Data Capture.
From www.slideserve.com
PPT The Use of Electronic Data Capture Tools in Clinical Trials What Is Electronic Data Capture what is edc in clinical trials? electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. Edc in clinical. What Is Electronic Data Capture.
From www.youtube.com
GCP Mindset Electronic Data Capture (EDC) YouTube What Is Electronic Data Capture what is edc in clinical trials? an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. Edc in clinical. What Is Electronic Data Capture.
From view.content4demand.com
10 Benefits of Electronic Data Capture 10 Benefits of Electronic What Is Electronic Data Capture Edc in clinical trials is a software system for collecting, managing, and storing data. what is edc in clinical trials? electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device. What Is Electronic Data Capture.
From www.clinion.com
Benefits of Electronic Data Capture System In Clinical Trials What Is Electronic Data Capture electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. what is edc in clinical trials? Edc in clinical. What Is Electronic Data Capture.
From allin-electronic.blogspot.com
Electronic Data Capture (edc) Definition Allin Electronic What Is Electronic Data Capture what is edc in clinical trials? electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. Edc in clinical trials is a software system for collecting, managing, and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device. What Is Electronic Data Capture.
From credevo.com
Best Electronic Data Capture (EDC) Software Credevo Articles What Is Electronic Data Capture electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. what is edc in clinical trials? Edc in clinical. What Is Electronic Data Capture.
From pepgra.com
Uses and Implementation process of Electronic Data Capture (EDC) in What Is Electronic Data Capture an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. what is edc in clinical trials? Edc in clinical trials is a software system for collecting, managing, and storing data. electronic data capture (edc). What Is Electronic Data Capture.
From www.datacapt.com
eCRF Electronic Data Capture Datacapt What Is Electronic Data Capture what is edc in clinical trials? Edc in clinical trials is a software system for collecting, managing, and storing data. electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device. What Is Electronic Data Capture.
From www.collidu.com
Electronic Data Capture PowerPoint Presentation Slides PPT Template What Is Electronic Data Capture what is edc in clinical trials? Edc in clinical trials is a software system for collecting, managing, and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. electronic data capture (edc). What Is Electronic Data Capture.
From www.researchgate.net
Simplified diagram illustrating the electronic data capture system What Is Electronic Data Capture what is edc in clinical trials? electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. Edc in clinical. What Is Electronic Data Capture.
From www.iconfinder.com
Edc, electronic, electronic data capture, computer, device, machine 3D What Is Electronic Data Capture what is edc in clinical trials? Edc in clinical trials is a software system for collecting, managing, and storing data. electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device. What Is Electronic Data Capture.
From allin-electronic.blogspot.com
Electronic Data Capture (edc) Definition Allin Electronic What Is Electronic Data Capture what is edc in clinical trials? electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. Edc in clinical trials is a software system for collecting, managing, and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device. What Is Electronic Data Capture.
From www.openclinica.com
Electronic Data Capture The Fundamentals of EDC OpenClinica What Is Electronic Data Capture electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. Edc in clinical trials is a software system for collecting,. What Is Electronic Data Capture.
From www.greenlight.guru
Electronic Data Capture (EDC) Systems What to Know What Is Electronic Data Capture electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. Edc in clinical trials is a software system for collecting, managing, and storing data. what is edc in clinical trials? an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device. What Is Electronic Data Capture.
From www.castoredc.com
An essential guide to Electronic Data Capture in clinical research What Is Electronic Data Capture what is edc in clinical trials? Edc in clinical trials is a software system for collecting, managing, and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. electronic data capture (edc). What Is Electronic Data Capture.
From www.linkedin.com
A Brief Look at Electronic Data Capture (EDC) in Clinical Trials What Is Electronic Data Capture an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. Edc in clinical trials is a software system for collecting, managing, and storing data. what is edc in clinical trials? electronic data capture (edc). What Is Electronic Data Capture.
From www.researchgate.net
Electronic data capture system for a multisite study. MySQLdefined What Is Electronic Data Capture electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. Edc in clinical trials is a software system for collecting,. What Is Electronic Data Capture.
From www.medidata.com
The Future of Electronic Data Capture and Clinical Data Management What Is Electronic Data Capture electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. what is edc in clinical trials? Edc in clinical. What Is Electronic Data Capture.
From www.socra.org
The Future of Clinical Trials Using Electronic Data Capture Systems SoCRA What Is Electronic Data Capture electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. what is edc in clinical trials? Edc in clinical trials is a software system for collecting, managing, and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device. What Is Electronic Data Capture.
From www.youtube.com
ResearchManager EDC Electronic Data Capture System to capture What Is Electronic Data Capture electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. Edc in clinical trials is a software system for collecting,. What Is Electronic Data Capture.
From www.collidu.com
Electronic Data Capture PowerPoint Presentation Slides PPT Template What Is Electronic Data Capture electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. what is edc in clinical trials? Edc in clinical trials is a software system for collecting, managing, and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device. What Is Electronic Data Capture.
From clinnovo.blogspot.com
Clinnovo News Electronic Data Capture (EDC) During Clinical Trials What Is Electronic Data Capture electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. Edc in clinical trials is a software system for collecting,. What Is Electronic Data Capture.
From www.collidu.com
Electronic Data Capture PowerPoint Presentation Slides PPT Template What Is Electronic Data Capture what is edc in clinical trials? Edc in clinical trials is a software system for collecting, managing, and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. electronic data capture (edc). What Is Electronic Data Capture.
From autodocs.co.uk
data capture Automated Document Services Ltd What Is Electronic Data Capture what is edc in clinical trials? Edc in clinical trials is a software system for collecting, managing, and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. electronic data capture (edc). What Is Electronic Data Capture.
From www.socra.org
The Future of Clinical Trials Using Electronic Data Capture Systems SoCRA What Is Electronic Data Capture what is edc in clinical trials? an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. Edc in clinical trials is a software system for collecting, managing, and storing data. electronic data capture (edc). What Is Electronic Data Capture.
From www.castoredc.com
Electronic Data Capture (EDC) Platform for Clinical Trials What Is Electronic Data Capture electronic data capture (edc) refers to the systematic process of collecting, managing and storing data. an electronic data capture (edc) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. what is edc in clinical trials? Edc in clinical. What Is Electronic Data Capture.