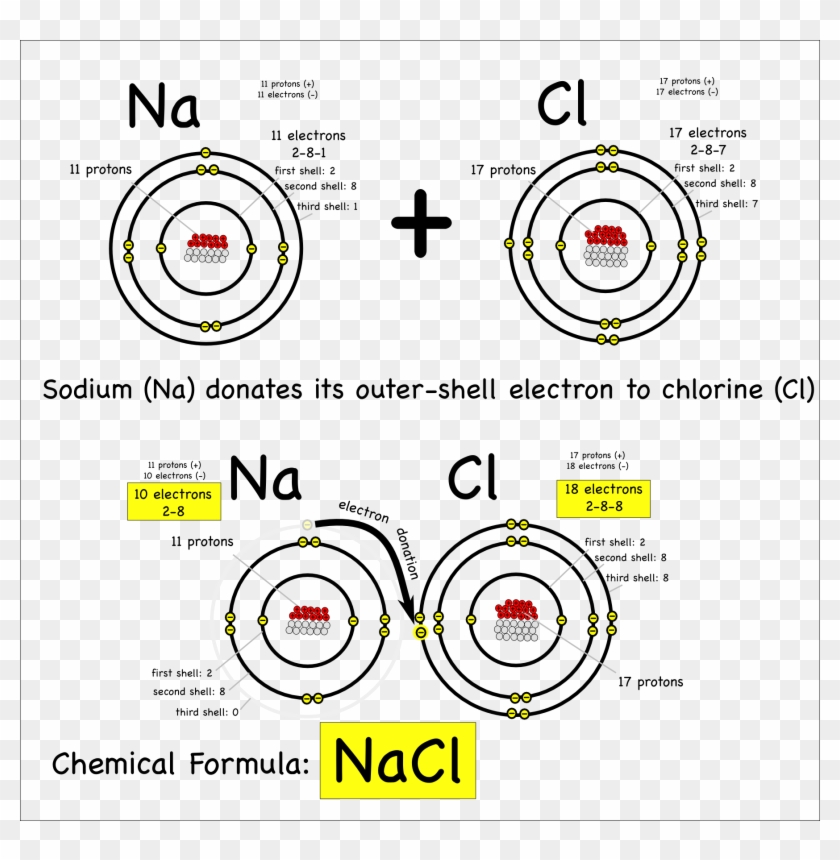
Why Chlorine Has High Electron Affinity Than . That is because although fluorine wants to attract. and yes, chlorine has a higher electron affinity than fluorine. fluorine, though higher than chlorine in the periodic table, has a very small atomic size. since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. It is an extremely reactive element and a strong oxidising. Since this periodic property tends from the bottom. why does chlorine have a higher electron affinity value than fluorine?
from giojcsdfs.blob.core.windows.net
why does chlorine have a higher electron affinity value than fluorine? fluorine, though higher than chlorine in the periodic table, has a very small atomic size. since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. and yes, chlorine has a higher electron affinity than fluorine. That is because although fluorine wants to attract. the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. It is an extremely reactive element and a strong oxidising. Since this periodic property tends from the bottom.
Chlorine Ionic Bond Form at Janyce Gallegos blog
Why Chlorine Has High Electron Affinity Than Since this periodic property tends from the bottom. since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. It is an extremely reactive element and a strong oxidising. Since this periodic property tends from the bottom. fluorine, though higher than chlorine in the periodic table, has a very small atomic size. and yes, chlorine has a higher electron affinity than fluorine. the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. why does chlorine have a higher electron affinity value than fluorine? That is because although fluorine wants to attract.
From slideplayer.com
The Periodic Table History Arrangement of Elements ppt download Why Chlorine Has High Electron Affinity Than and yes, chlorine has a higher electron affinity than fluorine. Since this periodic property tends from the bottom. since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. fluorine, though higher than chlorine in the periodic table, has a. Why Chlorine Has High Electron Affinity Than.
From giojcsdfs.blob.core.windows.net
Chlorine Ionic Bond Form at Janyce Gallegos blog Why Chlorine Has High Electron Affinity Than Since this periodic property tends from the bottom. and yes, chlorine has a higher electron affinity than fluorine. That is because although fluorine wants to attract. why does chlorine have a higher electron affinity value than fluorine? It is an extremely reactive element and a strong oxidising. fluorine, though higher than chlorine in the periodic table, has. Why Chlorine Has High Electron Affinity Than.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Why Chlorine Has High Electron Affinity Than why does chlorine have a higher electron affinity value than fluorine? since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. fluorine, though higher than chlorine in the periodic table, has a very small atomic size. It is an. Why Chlorine Has High Electron Affinity Than.
From slideplayer.com
Learning Objectives General trends of group 17 elements ppt download Why Chlorine Has High Electron Affinity Than Since this periodic property tends from the bottom. and yes, chlorine has a higher electron affinity than fluorine. fluorine, though higher than chlorine in the periodic table, has a very small atomic size. It is an extremely reactive element and a strong oxidising. That is because although fluorine wants to attract. the chlorine atom has the most. Why Chlorine Has High Electron Affinity Than.
From www.toppr.com
viii. Crystal radius is larger than covalent radius. ix. Electron Why Chlorine Has High Electron Affinity Than That is because although fluorine wants to attract. It is an extremely reactive element and a strong oxidising. the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. since chlorine's outermost orbital is a 3p orbital, there is more space, and the. Why Chlorine Has High Electron Affinity Than.
From slideplayer.com
CHEMICAL BONDING AND COMPOUND FORMATION ppt download Why Chlorine Has High Electron Affinity Than It is an extremely reactive element and a strong oxidising. the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. and yes, chlorine has a higher electron affinity than fluorine. That is because although fluorine wants to attract. why does chlorine. Why Chlorine Has High Electron Affinity Than.
From fyofgpkgr.blob.core.windows.net
Electron Affinity Of Chlorine Equation at Marilyn Burton blog Why Chlorine Has High Electron Affinity Than and yes, chlorine has a higher electron affinity than fluorine. why does chlorine have a higher electron affinity value than fluorine? the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. since chlorine's outermost orbital is a 3p orbital, there. Why Chlorine Has High Electron Affinity Than.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Why Chlorine Has High Electron Affinity Than the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. Since this periodic property tends from the bottom. That is because although fluorine wants to attract. since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in. Why Chlorine Has High Electron Affinity Than.
From www.toppr.com
Electronegativity of chlorine is three. Electron affinity of chlorine Why Chlorine Has High Electron Affinity Than the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. and yes, chlorine has a higher electron affinity than fluorine. It is an extremely reactive element and a strong oxidising. since chlorine's outermost orbital is a 3p orbital, there is more. Why Chlorine Has High Electron Affinity Than.
From www.numerade.com
SOLVED On the basis of electron configurations, explain why sulfur has Why Chlorine Has High Electron Affinity Than It is an extremely reactive element and a strong oxidising. why does chlorine have a higher electron affinity value than fluorine? Since this periodic property tends from the bottom. That is because although fluorine wants to attract. and yes, chlorine has a higher electron affinity than fluorine. since chlorine's outermost orbital is a 3p orbital, there is. Why Chlorine Has High Electron Affinity Than.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Why Chlorine Has High Electron Affinity Than Since this periodic property tends from the bottom. since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron. Why Chlorine Has High Electron Affinity Than.
From mungfali.com
Chlorine Orbital Diagram Why Chlorine Has High Electron Affinity Than since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. why does chlorine. Why Chlorine Has High Electron Affinity Than.
From brainly.in
Why chlorine has a higher electron affinity than fluorine? Brainly.in Why Chlorine Has High Electron Affinity Than It is an extremely reactive element and a strong oxidising. since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. why does chlorine have a higher electron affinity value than fluorine? fluorine, though higher than chlorine in the periodic. Why Chlorine Has High Electron Affinity Than.
From www.youtube.com
Why is electron affinity of chlorine more than thatof fluorine? CLASS Why Chlorine Has High Electron Affinity Than fluorine, though higher than chlorine in the periodic table, has a very small atomic size. the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. Since this periodic property tends from the bottom. since chlorine's outermost orbital is a 3p orbital,. Why Chlorine Has High Electron Affinity Than.
From www.doubtnut.com
Electron affinity of chlorine is larger than that of fluorine because Why Chlorine Has High Electron Affinity Than why does chlorine have a higher electron affinity value than fluorine? since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. That is because although fluorine wants to attract. It is an extremely reactive element and a strong oxidising. . Why Chlorine Has High Electron Affinity Than.
From www.meritnation.com
Does chlorine have higher electron affinity value than fluorine Why Chlorine Has High Electron Affinity Than the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. why does chlorine have a higher electron affinity value than fluorine? fluorine, though higher than chlorine in the periodic table, has a very small atomic size. and yes, chlorine has. Why Chlorine Has High Electron Affinity Than.
From chem.libretexts.org
Electron Affinity Chemistry LibreTexts Why Chlorine Has High Electron Affinity Than why does chlorine have a higher electron affinity value than fluorine? since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Since this periodic. Why Chlorine Has High Electron Affinity Than.
From www.collegesearch.in
Electron Affinity Factors Affecting, Halogens, Periodic Trends, Metals Why Chlorine Has High Electron Affinity Than Since this periodic property tends from the bottom. since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron. Why Chlorine Has High Electron Affinity Than.
From ecurrencythailand.com
Which Of The Following Has Highest Electron Affinity Of Fluorine Why Chlorine Has High Electron Affinity Than the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. It is an extremely reactive element and a strong oxidising. fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Since this periodic property tends from the. Why Chlorine Has High Electron Affinity Than.
From periodictableguide.com
Electron Affinity Chart (Labeled Periodic table + List) Why Chlorine Has High Electron Affinity Than fluorine, though higher than chlorine in the periodic table, has a very small atomic size. why does chlorine have a higher electron affinity value than fluorine? That is because although fluorine wants to attract. the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is. Why Chlorine Has High Electron Affinity Than.
From www.youtube.com
Electron affinity of chlorine is higher than that of flourine YouTube Why Chlorine Has High Electron Affinity Than and yes, chlorine has a higher electron affinity than fluorine. It is an extremely reactive element and a strong oxidising. That is because although fluorine wants to attract. the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. Since this periodic property. Why Chlorine Has High Electron Affinity Than.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Why Chlorine Has High Electron Affinity Than Since this periodic property tends from the bottom. since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron. Why Chlorine Has High Electron Affinity Than.
From www.youtube.com
Chlorine has ___________ electron affinity than fluorine. YouTube Why Chlorine Has High Electron Affinity Than and yes, chlorine has a higher electron affinity than fluorine. Since this periodic property tends from the bottom. It is an extremely reactive element and a strong oxidising. why does chlorine have a higher electron affinity value than fluorine? since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital. Why Chlorine Has High Electron Affinity Than.
From www.pinterest.co.kr
Electron Affinity Trend and Definition Electron affinity, Ionization Why Chlorine Has High Electron Affinity Than It is an extremely reactive element and a strong oxidising. since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. and yes, chlorine has a higher electron affinity than fluorine. fluorine, though higher than chlorine in the periodic table,. Why Chlorine Has High Electron Affinity Than.
From www.askiitians.com
Classification of Elements & Periodicity in Properties askIITians Why Chlorine Has High Electron Affinity Than That is because although fluorine wants to attract. since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. It is an extremely reactive element and a strong oxidising. Since this periodic property tends from the bottom. and yes, chlorine has. Why Chlorine Has High Electron Affinity Than.
From www.toppr.com
In the formation of a chloride ion, from an isolated gaseous chlorine Why Chlorine Has High Electron Affinity Than why does chlorine have a higher electron affinity value than fluorine? since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. That is because although fluorine wants to attract. and yes, chlorine has a higher electron affinity than fluorine.. Why Chlorine Has High Electron Affinity Than.
From www.numerade.com
SOLVEDUse electron configurations to explain why (a) sulfur has a Why Chlorine Has High Electron Affinity Than since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. and yes, chlorine has a higher electron affinity than fluorine. why does chlorine have a higher electron affinity value than fluorine? It is an extremely reactive element and a. Why Chlorine Has High Electron Affinity Than.
From chemistry.stackexchange.com
periodic trends If fluorine has a lower electron affinity than Why Chlorine Has High Electron Affinity Than and yes, chlorine has a higher electron affinity than fluorine. Since this periodic property tends from the bottom. since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. the chlorine atom has the most negative electron affinity of any. Why Chlorine Has High Electron Affinity Than.
From exosuznkq.blob.core.windows.net
Chlorine Has A Greater Electron Affinity Than Sodium at Janice Bourn blog Why Chlorine Has High Electron Affinity Than why does chlorine have a higher electron affinity value than fluorine? since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. the chlorine atom has the most negative electron affinity of any element, which means that more energy is. Why Chlorine Has High Electron Affinity Than.
From www.chegg.com
Solved Calcium has a greater electron affinity than Chlorine Why Chlorine Has High Electron Affinity Than fluorine, though higher than chlorine in the periodic table, has a very small atomic size. and yes, chlorine has a higher electron affinity than fluorine. why does chlorine have a higher electron affinity value than fluorine? since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined. Why Chlorine Has High Electron Affinity Than.
From brokeasshome.com
Periodic Table Chlorine Electrons Why Chlorine Has High Electron Affinity Than the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. Since this periodic property tends from the bottom. It is an extremely reactive element and a strong oxidising. That is because although fluorine wants to attract. and yes, chlorine has a higher. Why Chlorine Has High Electron Affinity Than.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of Why Chlorine Has High Electron Affinity Than why does chlorine have a higher electron affinity value than fluorine? That is because although fluorine wants to attract. It is an extremely reactive element and a strong oxidising. Since this periodic property tends from the bottom. the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an. Why Chlorine Has High Electron Affinity Than.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table Why Chlorine Has High Electron Affinity Than why does chlorine have a higher electron affinity value than fluorine? since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. and yes, chlorine has a higher electron affinity than fluorine. fluorine, though higher than chlorine in the. Why Chlorine Has High Electron Affinity Than.
From exosuznkq.blob.core.windows.net
Chlorine Has A Greater Electron Affinity Than Sodium at Janice Bourn blog Why Chlorine Has High Electron Affinity Than Since this periodic property tends from the bottom. It is an extremely reactive element and a strong oxidising. the chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a. and yes, chlorine has a higher electron affinity than fluorine. That is because although. Why Chlorine Has High Electron Affinity Than.
From www.toppr.com
The electron affinity of fluorine is less than that of chlorine because Why Chlorine Has High Electron Affinity Than since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. why does chlorine have a higher electron affinity value than fluorine? fluorine, though higher than chlorine in the periodic table, has a very small atomic size. That is because. Why Chlorine Has High Electron Affinity Than.