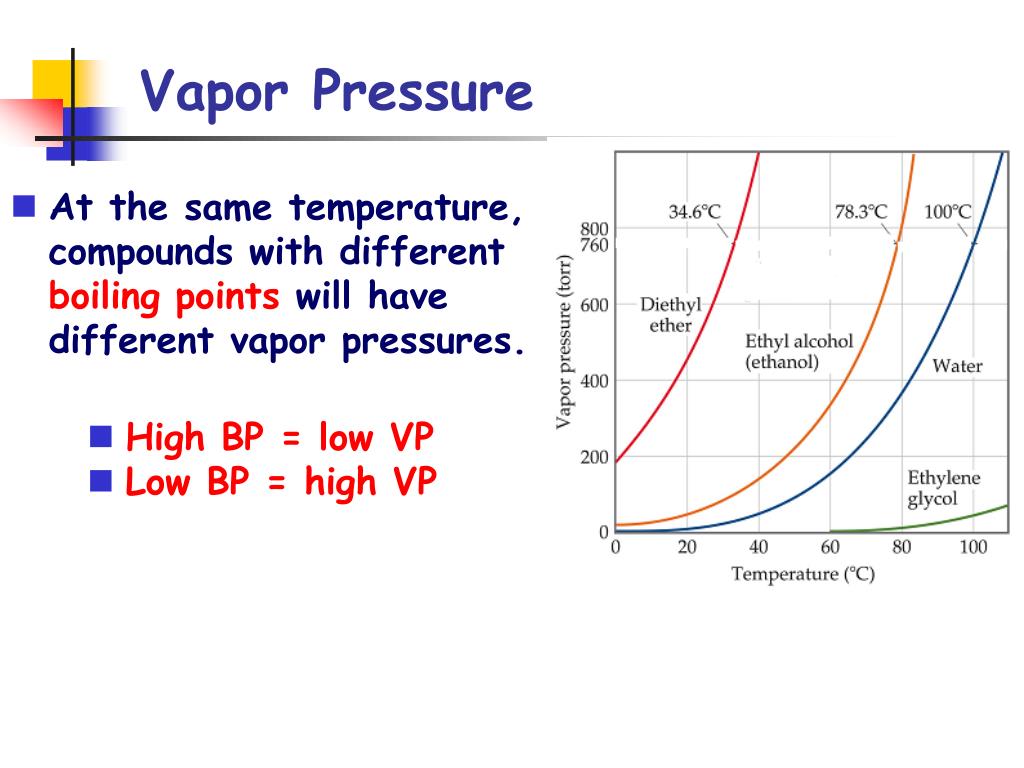
What Is Vapor Pressure Effect . That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); 80k views 3 years ago #vapor. Strong intermolecular forces produce a lower rate of evaporation and a. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e.
from www.slideserve.com
Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. 80k views 3 years ago #vapor. Strong intermolecular forces produce a lower rate of evaporation and a. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with.
PPT Phase Changes PowerPoint Presentation, free download ID2437650
What Is Vapor Pressure Effect Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. 80k views 3 years ago #vapor. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. Strong intermolecular forces produce a lower rate of evaporation and a.
From www.slideserve.com
PPT Liquids, Solids, and Intermolecular Forces PowerPoint What Is Vapor Pressure Effect 80k views 3 years ago #vapor. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Strong intermolecular forces produce a lower rate of evaporation and a. Vapor pressure is a measure of the. What Is Vapor Pressure Effect.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint What Is Vapor Pressure Effect That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Strong intermolecular forces produce a lower rate of evaporation and a. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature. What Is Vapor Pressure Effect.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 What Is Vapor Pressure Effect That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. The vapor pressure of a liquid is the. What Is Vapor Pressure Effect.
From www.godubrovnik.com
5 Effects of Vapor Pressure Deficit That Are Observed In Plants Go What Is Vapor Pressure Effect Strong intermolecular forces produce a lower rate of evaporation and a. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. 80k views 3 years ago #vapor. That is, the pressure of the vapor resulting from evaporation. What Is Vapor Pressure Effect.
From www.youtube.com
What is vapor pressure what is the vapor pressure of liquids What Is Vapor Pressure Effect Strong intermolecular forces produce a lower rate of evaporation and a. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. Vapor pressure is a measure of. What Is Vapor Pressure Effect.
From www.youtube.com
Vapor Pressure, Equilibrium Vapor Pressure, and Relative Humidity YouTube What Is Vapor Pressure Effect Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. 80k views 3 years ago #vapor. Strong intermolecular forces produce a lower rate of evaporation and a. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure. What Is Vapor Pressure Effect.
From evaaddperez.blogspot.com
What is Vapor Pressure EvaaddPerez What Is Vapor Pressure Effect As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its. What Is Vapor Pressure Effect.
From www.youtube.com
Vapor Pressure and Boiling YouTube What Is Vapor Pressure Effect That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. 80k views 3 years ago #vapor. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. Vapor pressure is a measure of. What Is Vapor Pressure Effect.
From www.researchgate.net
The effect of temperature on saturation vapor pressure, actual vapor What Is Vapor Pressure Effect Strong intermolecular forces produce a lower rate of evaporation and a. Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open.. What Is Vapor Pressure Effect.
From www.numerade.com
SOLVEDThis portion of a phase diagram shows the vaporpressure curves What Is Vapor Pressure Effect That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapour pressure, also known as vapour equilibrium pressure, can. What Is Vapor Pressure Effect.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint What Is Vapor Pressure Effect Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); 80k views 3 years ago #vapor. Strong intermolecular forces produce a lower rate of evaporation and a. Vapour pressure, also known as vapour equilibrium pressure, can be. What Is Vapor Pressure Effect.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 What Is Vapor Pressure Effect Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. Strong intermolecular forces produce a lower rate of evaporation and a. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. Generally a substance's vapor pressure. What Is Vapor Pressure Effect.
From study.com
Vapor Pressure Definition, Equation & Examples Video & Lesson What Is Vapor Pressure Effect That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or. What Is Vapor Pressure Effect.
From www.slideserve.com
PPT Why . . . PowerPoint Presentation, free download ID2250075 What Is Vapor Pressure Effect Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. Strong intermolecular forces produce a lower rate of evaporation and a. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Generally a substance's vapor pressure increases as temperature increases and decreases as. What Is Vapor Pressure Effect.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It What Is Vapor Pressure Effect 80k views 3 years ago #vapor. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. Strong intermolecular forces produce a lower rate of evaporation and. What Is Vapor Pressure Effect.
From socratic.org
Explain how each of the following affects the vapour pressure of a What Is Vapor Pressure Effect Strong intermolecular forces produce a lower rate of evaporation and a. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapor pressure is a. What Is Vapor Pressure Effect.
From byjus.com
What is the effect on vapour pressure of solution when external What Is Vapor Pressure Effect The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. As the temperature of a liquid increases, the vapor. What Is Vapor Pressure Effect.
From chemistryskills.com
Define and explain vapour pressure Chemistry Skills What Is Vapor Pressure Effect Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. As. What Is Vapor Pressure Effect.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Is Vapor Pressure Effect Strong intermolecular forces produce a lower rate of evaporation and a. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapor pressure is a measure of. What Is Vapor Pressure Effect.
From www.youtube.com
vapour pressure,definition,unit, factors YouTube What Is Vapor Pressure Effect Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals. What Is Vapor Pressure Effect.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID7038735 What Is Vapor Pressure Effect Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. Strong intermolecular forces produce a lower rate of evaporation and a. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. 80k views 3 years ago #vapor. As the temperature of a liquid. What Is Vapor Pressure Effect.
From ar.inspiredpencil.com
Vapor Pressure Example What Is Vapor Pressure Effect Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Strong intermolecular forces produce a lower rate of evaporation and a. 80k views 3 years ago #vapor. That is, the pressure of the. What Is Vapor Pressure Effect.
From www.slideserve.com
PPT Liquids and Solids PowerPoint Presentation, free download ID What Is Vapor Pressure Effect As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. 80k views 3 years ago #vapor. Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. Generally a substance's vapor pressure. What Is Vapor Pressure Effect.
From www.numerade.com
SOLVEDWhat is the effect of a nonvolatile solute on the vapor pressure What Is Vapor Pressure Effect Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. Strong intermolecular forces produce a lower rate of evaporation and a. 80k views 3 years ago #vapor. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); That is, the pressure of the. What Is Vapor Pressure Effect.
From www.slideserve.com
PPT Behavior of Gases PowerPoint Presentation, free download ID3483746 What Is Vapor Pressure Effect The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. Strong intermolecular forces produce a lower rate of evaporation and a. Vapour. What Is Vapor Pressure Effect.
From www.slideserve.com
PPT Why . . . PowerPoint Presentation, free download ID2250075 What Is Vapor Pressure Effect 80k views 3 years ago #vapor. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Strong intermolecular forces produce a lower rate of evaporation and a. Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. Vapour pressure, also known as vapour. What Is Vapor Pressure Effect.
From www.slideserve.com
PPT VAPOR PRESSURE OF WATER PowerPoint Presentation, free download What Is Vapor Pressure Effect That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. Strong intermolecular forces produce a lower rate of evaporation and a. 80k views 3 years ago #vapor. Vapour pressure, also known as vapour. What Is Vapor Pressure Effect.
From www.youtube.com
Colligative properties Vapor pressure lowering YouTube What Is Vapor Pressure Effect That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. As the temperature of a liquid increases, the vapor. What Is Vapor Pressure Effect.
From www.youtube.com
Vapour Pressure And Cavitation Basic Concepts Fluid Properties What Is Vapor Pressure Effect Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals. What Is Vapor Pressure Effect.
From engineerexcel.com
Vapor Pressure of Water Explained EngineerExcel What Is Vapor Pressure Effect Strong intermolecular forces produce a lower rate of evaporation and a. Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapor pressure is a measure of. What Is Vapor Pressure Effect.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube What Is Vapor Pressure Effect Strong intermolecular forces produce a lower rate of evaporation and a. 80k views 3 years ago #vapor. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. As the temperature of a liquid. What Is Vapor Pressure Effect.
From ecoursesonline.iasri.res.in
FM LESSON 3. PROPERTIES OF FLUID What Is Vapor Pressure Effect Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it. What Is Vapor Pressure Effect.
From saylordotorg.github.io
Vapor Pressure What Is Vapor Pressure Effect Vapour pressure, also known as vapour equilibrium pressure, can be defined as the pressure exerted (in a system featuring thermodynamic equilibrium) by a vapour with. 80k views 3 years ago #vapor. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The vapor pressure of a liquid is the equilibrium pressure of a vapor above. What Is Vapor Pressure Effect.
From webmis.highland.cc.il.us
Vapor Pressure What Is Vapor Pressure Effect Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid). What Is Vapor Pressure Effect.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 What Is Vapor Pressure Effect As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open. Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. The vapor pressure of a liquid is the equilibrium pressure of. What Is Vapor Pressure Effect.