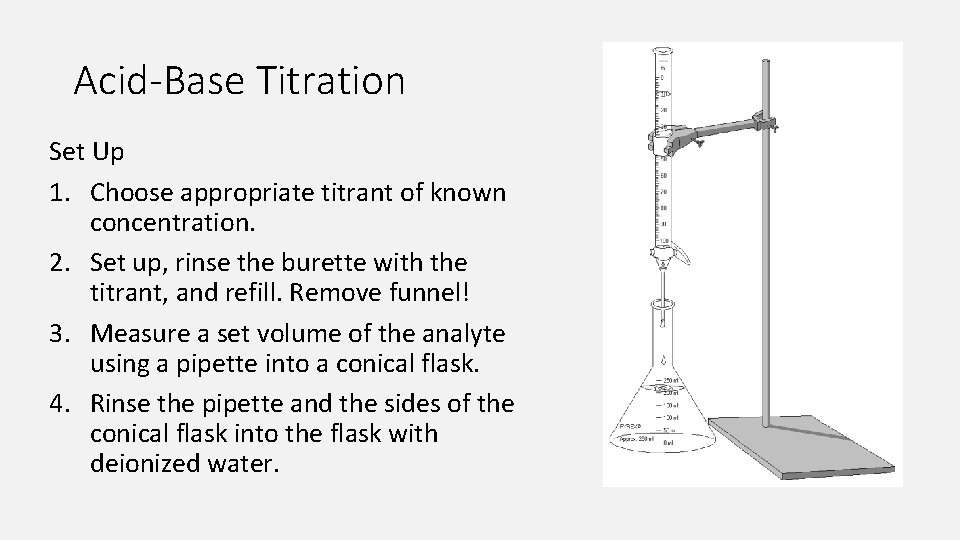
Your Titration And Quick Response . How would your ka values been affected if you overshot the titrations (meaning you added more naoh that you should have)?. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. The simplest way to track the progress of a titration is with the use of a chemical called an indicator. By the end of this section, you will be able to: You'll perform titration experiments to work out the. An indicator helps us to see the point of neutralisation during a. In level 1, you'll analyse samples from a river that has been contaminated with acid. A titration experiment can be used to determine the unknown concentration of acid using a known concentration of base.
from slidetodoc.com
A titration experiment can be used to determine the unknown concentration of acid using a known concentration of base. An indicator helps us to see the point of neutralisation during a. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. By the end of this section, you will be able to: The simplest way to track the progress of a titration is with the use of a chemical called an indicator. In level 1, you'll analyse samples from a river that has been contaminated with acid. You'll perform titration experiments to work out the. How would your ka values been affected if you overshot the titrations (meaning you added more naoh that you should have)?.
L 3 Titrations Learning Objectives Required Practical 1
Your Titration And Quick Response A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. A titration experiment can be used to determine the unknown concentration of acid using a known concentration of base. By the end of this section, you will be able to: How would your ka values been affected if you overshot the titrations (meaning you added more naoh that you should have)?. An indicator helps us to see the point of neutralisation during a. In level 1, you'll analyse samples from a river that has been contaminated with acid. You'll perform titration experiments to work out the. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. The simplest way to track the progress of a titration is with the use of a chemical called an indicator.
From www.youtube.com
Titrations Analytical Techniques Me Study YouTube Your Titration And Quick Response By the end of this section, you will be able to: The simplest way to track the progress of a titration is with the use of a chemical called an indicator. A titration experiment can be used to determine the unknown concentration of acid using a known concentration of base. A titration is a technique used in chemistry to help. Your Titration And Quick Response.
From scienceinfo.com
Redox titration Types, Applications, Advantages, Disadvantages Your Titration And Quick Response How would your ka values been affected if you overshot the titrations (meaning you added more naoh that you should have)?. An indicator helps us to see the point of neutralisation during a. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. You'll perform titration experiments to work. Your Titration And Quick Response.
From slidetodoc.com
L 3 Titrations Learning Objectives Required Practical 1 Your Titration And Quick Response In level 1, you'll analyse samples from a river that has been contaminated with acid. You'll perform titration experiments to work out the. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. By the end of this section, you will be able to: A titration experiment can be. Your Titration And Quick Response.
From www.showme.com
Topic Titrations ShowMe Online Learning Your Titration And Quick Response How would your ka values been affected if you overshot the titrations (meaning you added more naoh that you should have)?. By the end of this section, you will be able to: An indicator helps us to see the point of neutralisation during a. The simplest way to track the progress of a titration is with the use of a. Your Titration And Quick Response.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Your Titration And Quick Response The simplest way to track the progress of a titration is with the use of a chemical called an indicator. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. A titration experiment can be used to determine the unknown concentration of acid using a known concentration of base.. Your Titration And Quick Response.
From www.studocu.com
Titration Gizmo Student Exploration Titration Activity A Acids and Your Titration And Quick Response You'll perform titration experiments to work out the. An indicator helps us to see the point of neutralisation during a. The simplest way to track the progress of a titration is with the use of a chemical called an indicator. By the end of this section, you will be able to: A titration is a technique used in chemistry to. Your Titration And Quick Response.
From www.studocu.com
Titration Curves Interpretation of titration curves strong Acid Your Titration And Quick Response In level 1, you'll analyse samples from a river that has been contaminated with acid. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. By the end of this section, you will be able to: You'll perform titration experiments to work out the. How would your ka values. Your Titration And Quick Response.
From mavink.com
Titration Procedure Your Titration And Quick Response The simplest way to track the progress of a titration is with the use of a chemical called an indicator. An indicator helps us to see the point of neutralisation during a. You'll perform titration experiments to work out the. A titration experiment can be used to determine the unknown concentration of acid using a known concentration of base. A. Your Titration And Quick Response.
From www.studypool.com
SOLUTION Titrations titration meaning Studypool Your Titration And Quick Response A titration experiment can be used to determine the unknown concentration of acid using a known concentration of base. An indicator helps us to see the point of neutralisation during a. You'll perform titration experiments to work out the. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution.. Your Titration And Quick Response.
From www.chegg.com
Solved Match each titration curve with the appropriate Your Titration And Quick Response A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. By the end of this section, you will be able to: The simplest way to track the progress of a titration is with the use of a chemical called an indicator. In level 1, you'll analyse samples from a. Your Titration And Quick Response.
From www.numerade.com
SOLVED Match the provided labels to the appropriate point on the Your Titration And Quick Response A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. The simplest way to track the progress of a titration is with the use of a chemical called an indicator. How would your ka values been affected if you overshot the titrations (meaning you added more naoh that you. Your Titration And Quick Response.
From ar.inspiredpencil.com
Titration Setup Diagram Your Titration And Quick Response An indicator helps us to see the point of neutralisation during a. A titration experiment can be used to determine the unknown concentration of acid using a known concentration of base. The simplest way to track the progress of a titration is with the use of a chemical called an indicator. How would your ka values been affected if you. Your Titration And Quick Response.
From www.researchgate.net
Titrationresponse plots of SIEbla qHTS actives Titrationresponse Your Titration And Quick Response You'll perform titration experiments to work out the. An indicator helps us to see the point of neutralisation during a. In level 1, you'll analyse samples from a river that has been contaminated with acid. By the end of this section, you will be able to: The simplest way to track the progress of a titration is with the use. Your Titration And Quick Response.
From www.vectorstock.com
Acid base titration experiment and phases Vector Image Your Titration And Quick Response A titration experiment can be used to determine the unknown concentration of acid using a known concentration of base. The simplest way to track the progress of a titration is with the use of a chemical called an indicator. You'll perform titration experiments to work out the. An indicator helps us to see the point of neutralisation during a. In. Your Titration And Quick Response.
From mmerevise.co.uk
Titrations and Uncertainties MME Your Titration And Quick Response You'll perform titration experiments to work out the. In level 1, you'll analyse samples from a river that has been contaminated with acid. An indicator helps us to see the point of neutralisation during a. The simplest way to track the progress of a titration is with the use of a chemical called an indicator. How would your ka values. Your Titration And Quick Response.
From www.vrogue.co
14 3 Redox Reactions And Titrations Chemistry Librete vrogue.co Your Titration And Quick Response A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. The simplest way to track the progress of a titration is with the use of a chemical called an indicator. How would your ka values been affected if you overshot the titrations (meaning you added more naoh that you. Your Titration And Quick Response.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Your Titration And Quick Response A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. A titration experiment can be used to determine the unknown concentration of acid using a known concentration of base. The simplest way to track the progress of a titration is with the use of a chemical called an indicator.. Your Titration And Quick Response.
From www.homesciencetools.com
Titration Equipment Kit Learn How To Perform Titrations Your Titration And Quick Response You'll perform titration experiments to work out the. By the end of this section, you will be able to: The simplest way to track the progress of a titration is with the use of a chemical called an indicator. In level 1, you'll analyse samples from a river that has been contaminated with acid. A titration experiment can be used. Your Titration And Quick Response.
From byjus.com
Why is titration used? Your Titration And Quick Response A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. In level 1, you'll analyse samples from a river that has been contaminated with acid. An indicator helps us to see the point of neutralisation during a. By the end of this section, you will be able to: The. Your Titration And Quick Response.
From abhimcq360.medium.com
Top 20+ Titration MCQ Questions. Below are best multiple choice… by Your Titration And Quick Response An indicator helps us to see the point of neutralisation during a. The simplest way to track the progress of a titration is with the use of a chemical called an indicator. A titration experiment can be used to determine the unknown concentration of acid using a known concentration of base. How would your ka values been affected if you. Your Titration And Quick Response.
From www.slideserve.com
PPT Titrations! PowerPoint Presentation, free download ID4697719 Your Titration And Quick Response How would your ka values been affected if you overshot the titrations (meaning you added more naoh that you should have)?. By the end of this section, you will be able to: You'll perform titration experiments to work out the. A titration experiment can be used to determine the unknown concentration of acid using a known concentration of base. An. Your Titration And Quick Response.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID4319437 Your Titration And Quick Response An indicator helps us to see the point of neutralisation during a. A titration experiment can be used to determine the unknown concentration of acid using a known concentration of base. A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. You'll perform titration experiments to work out the.. Your Titration And Quick Response.
From www.hoddereducationmagazines.com
Performing the perfect titration Hodder Education Magazines Your Titration And Quick Response A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. An indicator helps us to see the point of neutralisation during a. By the end of this section, you will be able to: A titration experiment can be used to determine the unknown concentration of acid using a known. Your Titration And Quick Response.
From mmerevise.co.uk
Titrations and Uncertainties MME Your Titration And Quick Response The simplest way to track the progress of a titration is with the use of a chemical called an indicator. In level 1, you'll analyse samples from a river that has been contaminated with acid. An indicator helps us to see the point of neutralisation during a. How would your ka values been affected if you overshot the titrations (meaning. Your Titration And Quick Response.
From www.youtube.com
Quick Titration YouTube Your Titration And Quick Response A titration experiment can be used to determine the unknown concentration of acid using a known concentration of base. You'll perform titration experiments to work out the. In level 1, you'll analyse samples from a river that has been contaminated with acid. The simplest way to track the progress of a titration is with the use of a chemical called. Your Titration And Quick Response.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Your Titration And Quick Response In level 1, you'll analyse samples from a river that has been contaminated with acid. By the end of this section, you will be able to: A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. The simplest way to track the progress of a titration is with the. Your Titration And Quick Response.
From www.studocu.com
TITATRATION ACTIVITY Week 9 Unit Task Titration Illustrate and Your Titration And Quick Response How would your ka values been affected if you overshot the titrations (meaning you added more naoh that you should have)?. The simplest way to track the progress of a titration is with the use of a chemical called an indicator. You'll perform titration experiments to work out the. In level 1, you'll analyse samples from a river that has. Your Titration And Quick Response.
From knowledge.carolina.com
Titrations Techniques and Calculations Carolina Knowledge Center Your Titration And Quick Response In level 1, you'll analyse samples from a river that has been contaminated with acid. A titration experiment can be used to determine the unknown concentration of acid using a known concentration of base. How would your ka values been affected if you overshot the titrations (meaning you added more naoh that you should have)?. By the end of this. Your Titration And Quick Response.
From www.tes.com
Titrations Required Practical Sheet Teaching Resources Your Titration And Quick Response You'll perform titration experiments to work out the. By the end of this section, you will be able to: In level 1, you'll analyse samples from a river that has been contaminated with acid. A titration experiment can be used to determine the unknown concentration of acid using a known concentration of base. An indicator helps us to see the. Your Titration And Quick Response.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID5970774 Your Titration And Quick Response In level 1, you'll analyse samples from a river that has been contaminated with acid. You'll perform titration experiments to work out the. How would your ka values been affected if you overshot the titrations (meaning you added more naoh that you should have)?. By the end of this section, you will be able to: A titration experiment can be. Your Titration And Quick Response.
From www.studypool.com
SOLUTION Acid alkali and titration chemistry igcse edexcel notes 2 Your Titration And Quick Response By the end of this section, you will be able to: An indicator helps us to see the point of neutralisation during a. How would your ka values been affected if you overshot the titrations (meaning you added more naoh that you should have)?. The simplest way to track the progress of a titration is with the use of a. Your Titration And Quick Response.
From ar.inspiredpencil.com
Titration Diagram Your Titration And Quick Response By the end of this section, you will be able to: A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. You'll perform titration experiments to work out the. In level 1, you'll analyse samples from a river that has been contaminated with acid. An indicator helps us to. Your Titration And Quick Response.
From present5.com
Titration and AcidBase Neutralization LEARNING OBJECTIVES 11.2.2.7 Your Titration And Quick Response How would your ka values been affected if you overshot the titrations (meaning you added more naoh that you should have)?. By the end of this section, you will be able to: The simplest way to track the progress of a titration is with the use of a chemical called an indicator. A titration experiment can be used to determine. Your Titration And Quick Response.
From www.studocu.com
Worksheet Titration Tutorial 1 Titration Tutorial_Essay1_Course Your Titration And Quick Response A titration is a technique used in chemistry to help determine the concentration of a reactant mixed within an unknown solution. In level 1, you'll analyse samples from a river that has been contaminated with acid. How would your ka values been affected if you overshot the titrations (meaning you added more naoh that you should have)?. An indicator helps. Your Titration And Quick Response.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Your Titration And Quick Response In level 1, you'll analyse samples from a river that has been contaminated with acid. By the end of this section, you will be able to: An indicator helps us to see the point of neutralisation during a. How would your ka values been affected if you overshot the titrations (meaning you added more naoh that you should have)?. The. Your Titration And Quick Response.