Manufacturing Qualification . Principles of qualification and validation. Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline for subsequent. Operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. Full list of training courses by topic. Manufacturer’s overall philosophy and approach, to be used for establishing performance adequacy. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal drug and biological. It provides information on the manufacturer’s. In stage 2, the manufacturing process is.
from ink-safety-portal.siegwerk.com
Operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline for subsequent. Principles of qualification and validation. In stage 2, the manufacturing process is. Manufacturer’s overall philosophy and approach, to be used for establishing performance adequacy. It provides information on the manufacturer’s. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal drug and biological. Full list of training courses by topic.
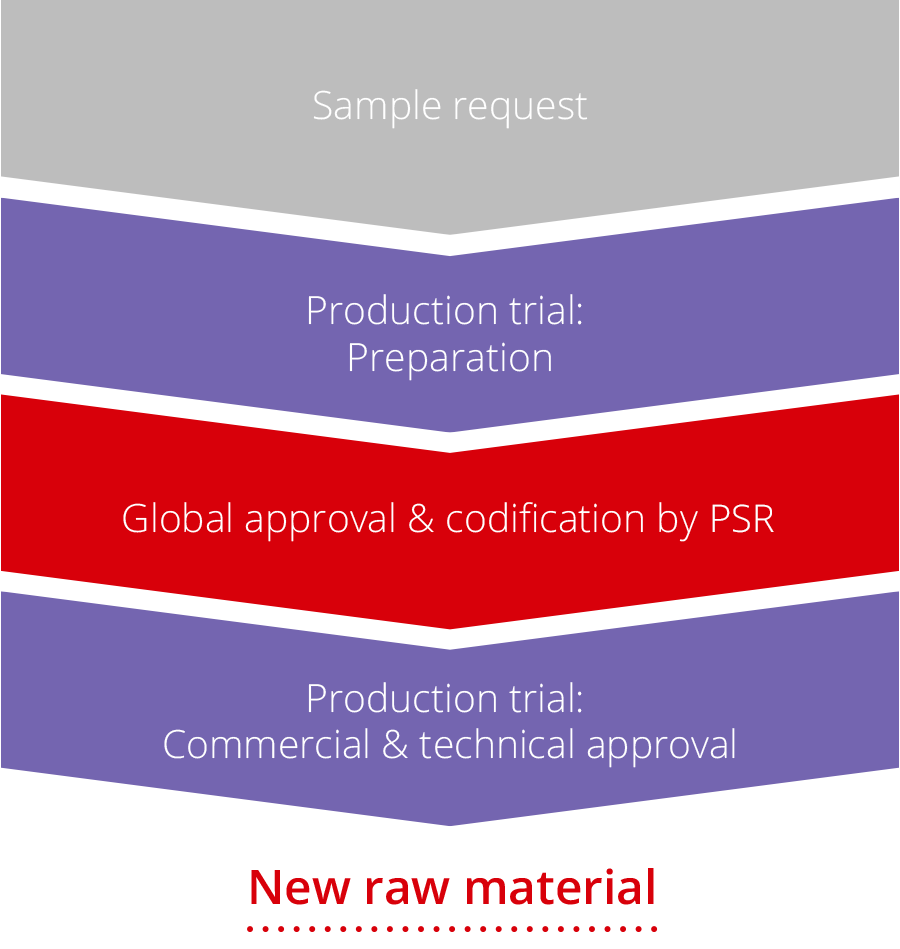
Raw Material Qualification Siegwerk
Manufacturing Qualification In stage 2, the manufacturing process is. In stage 2, the manufacturing process is. Operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. Full list of training courses by topic. Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline for subsequent. Principles of qualification and validation. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal drug and biological. Manufacturer’s overall philosophy and approach, to be used for establishing performance adequacy. It provides information on the manufacturer’s.
From slidetodoc.com
Qualification And Validation In Pharmaceutical Manufacturing KHADIJAH Manufacturing Qualification Manufacturer’s overall philosophy and approach, to be used for establishing performance adequacy. Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline for subsequent. Operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. In stage 2, the manufacturing process is. This guidance outlines the general principles and approaches. Manufacturing Qualification.
From vimeo.com
WEBINAR How to Earn an Additive Manufacturing Qualification in the Manufacturing Qualification This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal drug and biological. Operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. Manufacturer’s overall philosophy and approach, to be used for establishing performance adequacy. Full list of training courses by topic.. Manufacturing Qualification.
From slidetodoc.com
Qualification And Validation In Pharmaceutical Manufacturing KHADIJAH Manufacturing Qualification Operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. Principles of qualification and validation. In stage 2, the manufacturing process is. Full list of training courses by topic. It provides information on the manufacturer’s. Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline for subsequent. Manufacturer’s overall. Manufacturing Qualification.
From www.barnesglobaladvisors.com
The Barnes Global Advisors Additive Manufacturing Consulting Manufacturing Qualification In stage 2, the manufacturing process is. Manufacturer’s overall philosophy and approach, to be used for establishing performance adequacy. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal drug and biological. Operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality.. Manufacturing Qualification.
From www.dukascompressor.com
Qualification Certificate Shandong Dukas Machinery Manufacturing Co Manufacturing Qualification Manufacturer’s overall philosophy and approach, to be used for establishing performance adequacy. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal drug and biological. Operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. In stage 2, the manufacturing process is.. Manufacturing Qualification.
From www.getreskilled.com
Commissioning vs Qualification vs Validation in Pharma GetReskilled Manufacturing Qualification Full list of training courses by topic. It provides information on the manufacturer’s. In stage 2, the manufacturing process is. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal drug and biological. Qualification testing is performed to verify the design and manufacturing process, and it provides. Manufacturing Qualification.
From sitemate.com
Procedure Qualification Record (PQR) template Digital format or PDF Manufacturing Qualification In stage 2, the manufacturing process is. Operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline for subsequent. It provides information on the manufacturer’s. Principles of qualification and validation. Full list of training courses by topic. Manufacturer’s overall. Manufacturing Qualification.
From www.researchgate.net
The process of qualification and certification with the steps of raw Manufacturing Qualification Full list of training courses by topic. Manufacturer’s overall philosophy and approach, to be used for establishing performance adequacy. In stage 2, the manufacturing process is. Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline for subsequent. It provides information on the manufacturer’s. Operational qualification (oq) involves identifying and inspecting equipment features that. Manufacturing Qualification.
From www.getreskilled.com
Qualification vs Validation in Pharma GetReskilled Manufacturing Qualification This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal drug and biological. In stage 2, the manufacturing process is. It provides information on the manufacturer’s. Principles of qualification and validation. Manufacturer’s overall philosophy and approach, to be used for establishing performance adequacy. Qualification testing is performed. Manufacturing Qualification.
From www.slideteam.net
Manufacturing Quality Management Software Comparison Matrix Manufacturing Qualification In stage 2, the manufacturing process is. Manufacturer’s overall philosophy and approach, to be used for establishing performance adequacy. Operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline for subsequent. It provides information on the manufacturer’s. This guidance. Manufacturing Qualification.
From www.researchgate.net
(PDF) Qualification Challenges with Additive Manufacturing in Space Manufacturing Qualification Full list of training courses by topic. In stage 2, the manufacturing process is. It provides information on the manufacturer’s. Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline for subsequent. Principles of qualification and validation. Manufacturer’s overall philosophy and approach, to be used for establishing performance adequacy. Operational qualification (oq) involves identifying. Manufacturing Qualification.
From www.additivemanufacturing.media
Additive Manufacturing for Defense Targeting Qualification Additive Manufacturing Qualification Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline for subsequent. Manufacturer’s overall philosophy and approach, to be used for establishing performance adequacy. Principles of qualification and validation. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal drug and. Manufacturing Qualification.
From www.complianceteamllc.com
How To Move, Qualify & Validate Your Manufacturing Facility And Equipment Manufacturing Qualification In stage 2, the manufacturing process is. It provides information on the manufacturer’s. Manufacturer’s overall philosophy and approach, to be used for establishing performance adequacy. Full list of training courses by topic. Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline for subsequent. This guidance outlines the general principles and approaches that fda. Manufacturing Qualification.
From aidro.it
Aidro achieves Additive Manufacturing Qualification from DNV Aidro Manufacturing Qualification Full list of training courses by topic. Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline for subsequent. Operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. Manufacturer’s overall philosophy and approach, to be used for establishing performance adequacy. It provides information on the manufacturer’s. In stage. Manufacturing Qualification.
From jnfittings.com
QUALIFICATION JN PIPING & EQUIPMENT CO., LTD. Manufacturing Qualification Operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. It provides information on the manufacturer’s. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal drug and biological. Qualification testing is performed to verify the design and manufacturing process, and it. Manufacturing Qualification.
From expressionscoastalgifts.com
Certificate Of Manufacture Template 4 Best Templates Ideas Manufacturing Qualification It provides information on the manufacturer’s. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal drug and biological. Operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. Manufacturer’s overall philosophy and approach, to be used for establishing performance adequacy. In. Manufacturing Qualification.
From slidetodoc.com
Qualification And Validation In Pharmaceutical Manufacturing KHADIJAH Manufacturing Qualification Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline for subsequent. Manufacturer’s overall philosophy and approach, to be used for establishing performance adequacy. Full list of training courses by topic. Operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. Principles of qualification and validation. It provides information. Manufacturing Qualification.
From www.eos.info
An InDepth Look Into Additive Manufacturing Part Qualification Manufacturing Qualification Operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. It provides information on the manufacturer’s. Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline for subsequent. Full list of training courses by topic. In stage 2, the manufacturing process is. This guidance outlines the general principles and. Manufacturing Qualification.
From www.alamy.com
Qualification certificate hires stock photography and images Alamy Manufacturing Qualification Principles of qualification and validation. Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline for subsequent. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal drug and biological. Full list of training courses by topic. In stage 2, the. Manufacturing Qualification.
From www.amchronicle.com
EWF launches additive manufacturing qualification system for operators Manufacturing Qualification This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal drug and biological. In stage 2, the manufacturing process is. Operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. It provides information on the manufacturer’s. Qualification testing is performed to verify. Manufacturing Qualification.
From kvalito.ch
Commissioning, Qualification, and Validation (CQV) for Facility Manufacturing Qualification Principles of qualification and validation. Manufacturer’s overall philosophy and approach, to be used for establishing performance adequacy. In stage 2, the manufacturing process is. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal drug and biological. Operational qualification (oq) involves identifying and inspecting equipment features that. Manufacturing Qualification.
From www.researchgate.net
A broad representation of AM qualification flow about the material Manufacturing Qualification In stage 2, the manufacturing process is. Operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. Principles of qualification and validation. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal drug and biological. Manufacturer’s overall philosophy and approach, to be. Manufacturing Qualification.
From www.credly.com
TBGA Essentials of Qualification and Certification for Additive Manufacturing Qualification Manufacturer’s overall philosophy and approach, to be used for establishing performance adequacy. It provides information on the manufacturer’s. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal drug and biological. Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline. Manufacturing Qualification.
From www.en-standard.eu
PD CEN ISO/ASTM/TS 529302021 Additive Manufacturing. Qualification Manufacturing Qualification Operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline for subsequent. It provides information on the manufacturer’s. Manufacturer’s overall philosophy and approach, to be used for establishing performance adequacy. Full list of training courses by topic. This guidance. Manufacturing Qualification.
From www.yumpu.com
LG Manufacturer's Certification Statement Manufacturing Qualification In stage 2, the manufacturing process is. Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline for subsequent. Operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. Full list of training courses by topic. Principles of qualification and validation. It provides information on the manufacturer’s. This guidance. Manufacturing Qualification.
From www.slideteam.net
Steps To Setup Effective Quality Management System For Manufacturing Manufacturing Qualification Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline for subsequent. Full list of training courses by topic. It provides information on the manufacturer’s. Operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. This guidance outlines the general principles and approaches that fda considers appropriate elements of. Manufacturing Qualification.
From iqs.com.tr
Welded Manufacturing Qualification Certification IQS Manufacturing Qualification In stage 2, the manufacturing process is. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal drug and biological. Principles of qualification and validation. It provides information on the manufacturer’s. Operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. Qualification. Manufacturing Qualification.
From www.akmermerboru.com.tr
Manufacturing Qualification Certificate (ASO), AKMERMER BORU; SPIRAL Manufacturing Qualification Operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. It provides information on the manufacturer’s. Full list of training courses by topic. In stage 2, the manufacturing process is. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal drug and. Manufacturing Qualification.
From ioc.xtec.cat
Anglès Manufacturing Qualification Manufacturer’s overall philosophy and approach, to be used for establishing performance adequacy. In stage 2, the manufacturing process is. Full list of training courses by topic. It provides information on the manufacturer’s. Principles of qualification and validation. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal. Manufacturing Qualification.
From www.linkedin.com
Qualification Testing in Manufacturing Ensuring Quality and Reliability Manufacturing Qualification Principles of qualification and validation. Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline for subsequent. Operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and. Manufacturing Qualification.
From www.slideserve.com
PPT EQUIPMENT AND ITS QUALIFICATION PowerPoint Presentation, free Manufacturing Qualification It provides information on the manufacturer’s. Operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. In stage 2, the manufacturing process is. Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline for subsequent. This guidance outlines the general principles and approaches that fda considers appropriate elements of. Manufacturing Qualification.
From ink-safety-portal.siegwerk.com
Raw Material Qualification Siegwerk Manufacturing Qualification This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal drug and biological. Manufacturer’s overall philosophy and approach, to be used for establishing performance adequacy. It provides information on the manufacturer’s. Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline. Manufacturing Qualification.
From www.studypool.com
SOLUTION Commissioning qualification and validation supplementary Manufacturing Qualification Manufacturer’s overall philosophy and approach, to be used for establishing performance adequacy. Full list of training courses by topic. It provides information on the manufacturer’s. Principles of qualification and validation. Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline for subsequent. In stage 2, the manufacturing process is. Operational qualification (oq) involves identifying. Manufacturing Qualification.
From www.business-in-a-box.com
Manufacturing Quality Agreement Template by BusinessinaBox™ Manufacturing Qualification Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline for subsequent. Manufacturer’s overall philosophy and approach, to be used for establishing performance adequacy. In stage 2, the manufacturing process is. It provides information on the manufacturer’s. Full list of training courses by topic. Operational qualification (oq) involves identifying and inspecting equipment features that. Manufacturing Qualification.
From jialitxk.en.made-in-china.com
TUV certificate for manufacturer JIALI MACHINERY CO., LTD. Manufacturing Qualification This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal drug and biological. Manufacturer’s overall philosophy and approach, to be used for establishing performance adequacy. Qualification testing is performed to verify the design and manufacturing process, and it provides a baseline for subsequent. Full list of training. Manufacturing Qualification.