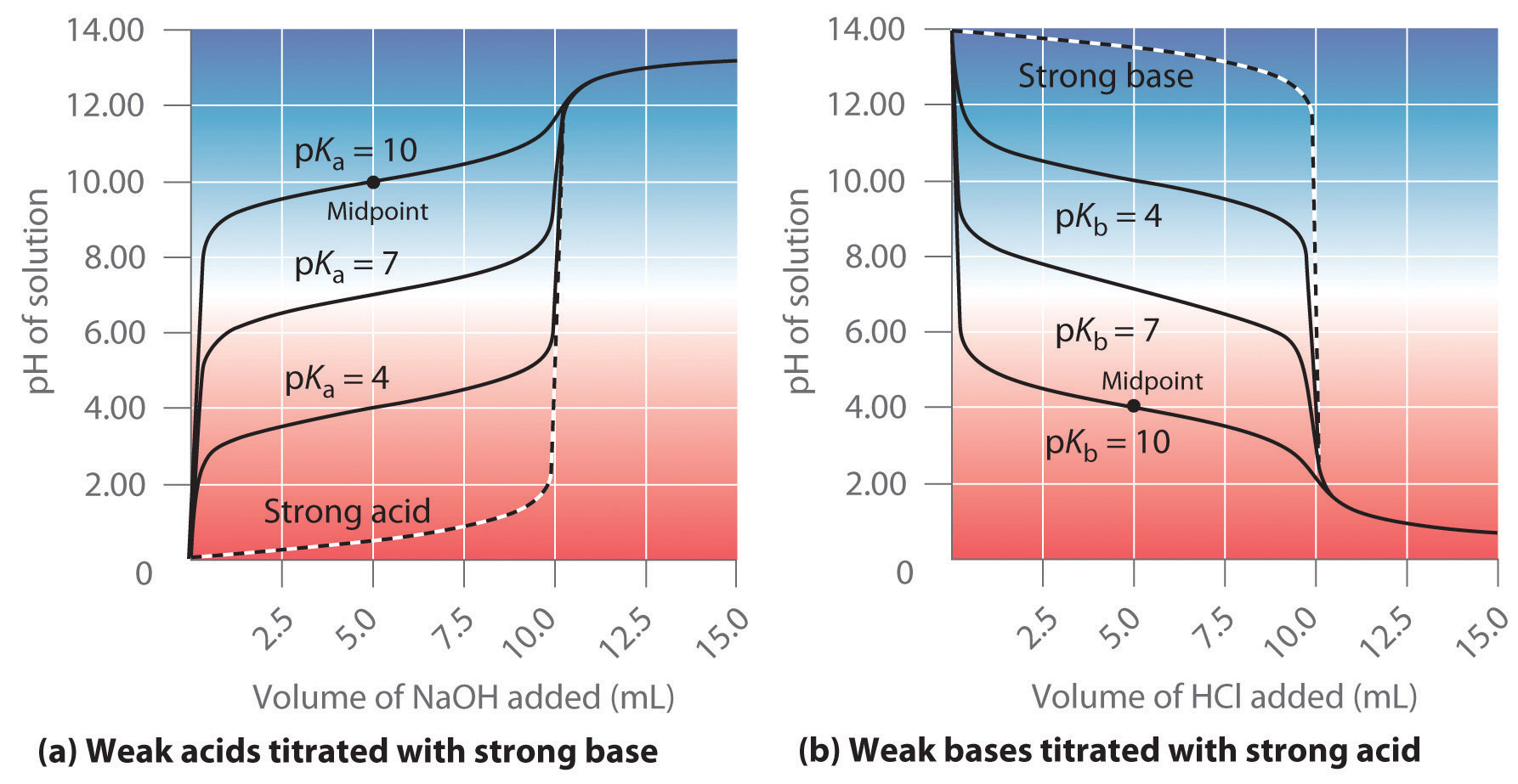
How To Do Acid Base Titration . a titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. how to do acid base titration? the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. there are two basic types of acid base titrations, indicator and potentiometric. the process of obtaining quantitative information of a sample using a fast chemical reaction by reacting with a. The analyte (titrand) is the solution with an unknown molarity. any introductory chemistry class will include titrations, and to do. titration is a common laboratory technique for determining the concentration of a solute. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence. a titration curve is a plot of some solution property versus the amount of added titrant. The reagent (titrant) is the solution with a known molarity that will react with the analyte.
from webmis.highland.cc.il.us
At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. how to do acid base titration? titration is a common laboratory technique for determining the concentration of a solute. a titration curve is a plot of some solution property versus the amount of added titrant. the process of obtaining quantitative information of a sample using a fast chemical reaction by reacting with a. any introductory chemistry class will include titrations, and to do. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. there are two basic types of acid base titrations, indicator and potentiometric. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence.
AcidBase Titrations
How To Do Acid Base Titration At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. there are two basic types of acid base titrations, indicator and potentiometric. any introductory chemistry class will include titrations, and to do. titration is a common laboratory technique for determining the concentration of a solute. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. how to do acid base titration? the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence. a titration curve is a plot of some solution property versus the amount of added titrant. The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. a titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. the process of obtaining quantitative information of a sample using a fast chemical reaction by reacting with a.
From scienceinfo.com
Acidbase Titration 4 Types, Theory, Working Principle How To Do Acid Base Titration there are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand) is the solution with an unknown molarity. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence. the titration of a weak acid with a strong base involves the direct transfer of. How To Do Acid Base Titration.
From www.biorender.com
AcidBase Titration BioRender Science Templates How To Do Acid Base Titration a titration curve is a plot of some solution property versus the amount of added titrant. the process of obtaining quantitative information of a sample using a fast chemical reaction by reacting with a. titration is a common laboratory technique for determining the concentration of a solute. how to do acid base titration? It is based. How To Do Acid Base Titration.
From www.youtube.com
Titration Weak Acid Strong Base Equivalence Point YouTube How To Do Acid Base Titration In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence. a titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. how to do acid base titration? the titration of a weak acid with a strong base involves the. How To Do Acid Base Titration.
From ar.inspiredpencil.com
Diagram Of Acid Base Titration How To Do Acid Base Titration In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence. The analyte (titrand) is the solution with an unknown molarity. how to do acid base titration? a titration curve is a plot of some solution property versus the amount of added titrant. a titration can be. How To Do Acid Base Titration.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube How To Do Acid Base Titration The reagent (titrant) is the solution with a known molarity that will react with the analyte. the process of obtaining quantitative information of a sample using a fast chemical reaction by reacting with a. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a titration curve is a plot. How To Do Acid Base Titration.
From mungfali.com
Acid Base Titration Lab How To Do Acid Base Titration the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. The analyte (titrand) is the solution with an unknown molarity. a titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. any introductory chemistry class will include titrations, and. How To Do Acid Base Titration.
From webmis.highland.cc.il.us
AcidBase Titrations How To Do Acid Base Titration titration is a common laboratory technique for determining the concentration of a solute. there are two basic types of acid base titrations, indicator and potentiometric. any introductory chemistry class will include titrations, and to do. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a titration curve. How To Do Acid Base Titration.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps How To Do Acid Base Titration how to do acid base titration? The reagent (titrant) is the solution with a known molarity that will react with the analyte. there are two basic types of acid base titrations, indicator and potentiometric. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. a. How To Do Acid Base Titration.
From parkermcyrandolph.blogspot.com
Acid Base Titration Lab Report ParkermcyRandolph How To Do Acid Base Titration the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. there are two basic types of acid base titrations, indicator and potentiometric. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a titration can be performed with. How To Do Acid Base Titration.
From www.vecteezy.com
Acid base titration experiment and phases of color change during How To Do Acid Base Titration the process of obtaining quantitative information of a sample using a fast chemical reaction by reacting with a. how to do acid base titration? a titration curve is a plot of some solution property versus the amount of added titrant. the titration of a weak acid with a strong base involves the direct transfer of protons. How To Do Acid Base Titration.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps How To Do Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence. there are two basic types of acid base titrations, indicator and potentiometric. the process of obtaining quantitative information of a sample using a fast chemical reaction by. How To Do Acid Base Titration.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps How To Do Acid Base Titration the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. any introductory chemistry class will include titrations, and to do. The analyte (titrand) is the solution with an unknown molarity. a titration curve is a plot of some solution property versus the amount of added titrant.. How To Do Acid Base Titration.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom How To Do Acid Base Titration It is based on the neutralization reaction, where an acid and a base react to form water and a salt. the process of obtaining quantitative information of a sample using a fast chemical reaction by reacting with a. The reagent (titrant) is the solution with a known molarity that will react with the analyte. At the equivalence point in. How To Do Acid Base Titration.
From mungfali.com
Acid Base Titration Calculation How To Do Acid Base Titration a titration curve is a plot of some solution property versus the amount of added titrant. titration is a common laboratory technique for determining the concentration of a solute. the process of obtaining quantitative information of a sample using a fast chemical reaction by reacting with a. how to do acid base titration? there are. How To Do Acid Base Titration.
From saylordotorg.github.io
AcidBase Titrations How To Do Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. there are two basic types of acid base titrations, indicator and potentiometric.. How To Do Acid Base Titration.
From mungfali.com
Acid Base Titration Graph How To Do Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. The reagent (titrant) is the solution with a known molarity that will react with the analyte. how to do acid base titration? any introductory chemistry class will include. How To Do Acid Base Titration.
From www.youtube.com
AcidBase Titrations Calculating Concentration of a Standard Solution How To Do Acid Base Titration a titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. the process of obtaining quantitative information of a sample using a fast chemical reaction by reacting with a. a titration curve is a plot of some solution property versus the amount of added titrant. there are two basic. How To Do Acid Base Titration.
From mungfali.com
Acid Base Titration Calculation How To Do Acid Base Titration It is based on the neutralization reaction, where an acid and a base react to form water and a salt. The reagent (titrant) is the solution with a known molarity that will react with the analyte. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence. At the equivalence. How To Do Acid Base Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators How To Do Acid Base Titration At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The reagent (titrant) is the solution with a known molarity that will react with the analyte. titration is a common laboratory technique for determining the concentration of a solute. the process of obtaining quantitative information of a sample using a. How To Do Acid Base Titration.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts How To Do Acid Base Titration the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. In a ph titration you measure the ph as a function of the volume of titrant added and. How To Do Acid Base Titration.
From saylordotorg.github.io
AcidBase Titrations How To Do Acid Base Titration the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. It is based on the neutralization reaction, where an acid and a base react to form water and a salt.. How To Do Acid Base Titration.
From www.aiophotoz.com
Difference Between Acid Base Titration And Redox Titration Acid Base How To Do Acid Base Titration At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The analyte (titrand) is the solution with an unknown molarity. the process of obtaining quantitative information of a sample using a fast chemical reaction by reacting with a. there are two basic types of acid base titrations, indicator and potentiometric.. How To Do Acid Base Titration.
From www.flinnsci.com
AcidBase Titrations—Classic Lab Kit for AP® Chemistry Flinn Scientific How To Do Acid Base Titration any introductory chemistry class will include titrations, and to do. a titration curve is a plot of some solution property versus the amount of added titrant. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. how to do acid base titration? In a ph. How To Do Acid Base Titration.
From www.dreamstime.com
Phenolphthalein Indicator in Acidbase Titration Stock Vector How To Do Acid Base Titration any introductory chemistry class will include titrations, and to do. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. titration is a common laboratory technique for determining the concentration of a solute. In a ph titration you measure the ph as a function of the. How To Do Acid Base Titration.
From chemistry.stackexchange.com
equilibrium How to calculate the dissociation constant of a weak acid How To Do Acid Base Titration there are two basic types of acid base titrations, indicator and potentiometric. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. a titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. a titration curve is a plot of. How To Do Acid Base Titration.
From www.thinkswap.com
Complete Acid/Base Titration Report Chemistry Year 12 HSC Thinkswap How To Do Acid Base Titration At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. titration is a common laboratory technique for determining the concentration of a solute. a titration curve is a plot of some. How To Do Acid Base Titration.
From www.vecteezy.com
Acid base titration experiment and phases of color change during How To Do Acid Base Titration any introductory chemistry class will include titrations, and to do. a titration curve is a plot of some solution property versus the amount of added titrant. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. The analyte (titrand) is the solution with an unknown molarity. titration. How To Do Acid Base Titration.
From www.sciencephoto.com
AcidBase Titration Stock Image C002/8122 Science Photo Library How To Do Acid Base Titration The reagent (titrant) is the solution with a known molarity that will react with the analyte. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. any introductory chemistry class will include titrations, and to do. titration is a common laboratory technique for determining the concentration. How To Do Acid Base Titration.
From mungfali.com
Acid Base Titration Calculation How To Do Acid Base Titration any introductory chemistry class will include titrations, and to do. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence. a titration curve is a plot of some solution property versus the amount of added titrant. the process of obtaining quantitative information of a sample using. How To Do Acid Base Titration.
From www.youtube.com
Solving AcidBase Titration Problems YouTube How To Do Acid Base Titration the process of obtaining quantitative information of a sample using a fast chemical reaction by reacting with a. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. In a ph titration you measure the ph as a function of the volume of titrant added and determine. How To Do Acid Base Titration.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co How To Do Acid Base Titration any introductory chemistry class will include titrations, and to do. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. a titration can be performed. How To Do Acid Base Titration.
From www.sliderbase.com
Titration How To Do Acid Base Titration the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. The analyte (titrand) is the solution with an unknown molarity. how to do acid base titration? a titration curve is a plot of some solution property versus the amount of added titrant. At the equivalence point. How To Do Acid Base Titration.
From www.chemistrylearner.com
Free Printable Acids and Bases Titration Worksheets How To Do Acid Base Titration At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The reagent (titrant) is the solution with a known molarity that will react with the analyte. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. In a ph titration. How To Do Acid Base Titration.
From mavink.com
Types Of Acid Base Titration How To Do Acid Base Titration At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence. . How To Do Acid Base Titration.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators How To Do Acid Base Titration a titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. titration is a common laboratory technique for determining the concentration of a solute. there are two basic types of acid base titrations, indicator and potentiometric. the titration of a weak acid with a strong base involves the direct. How To Do Acid Base Titration.