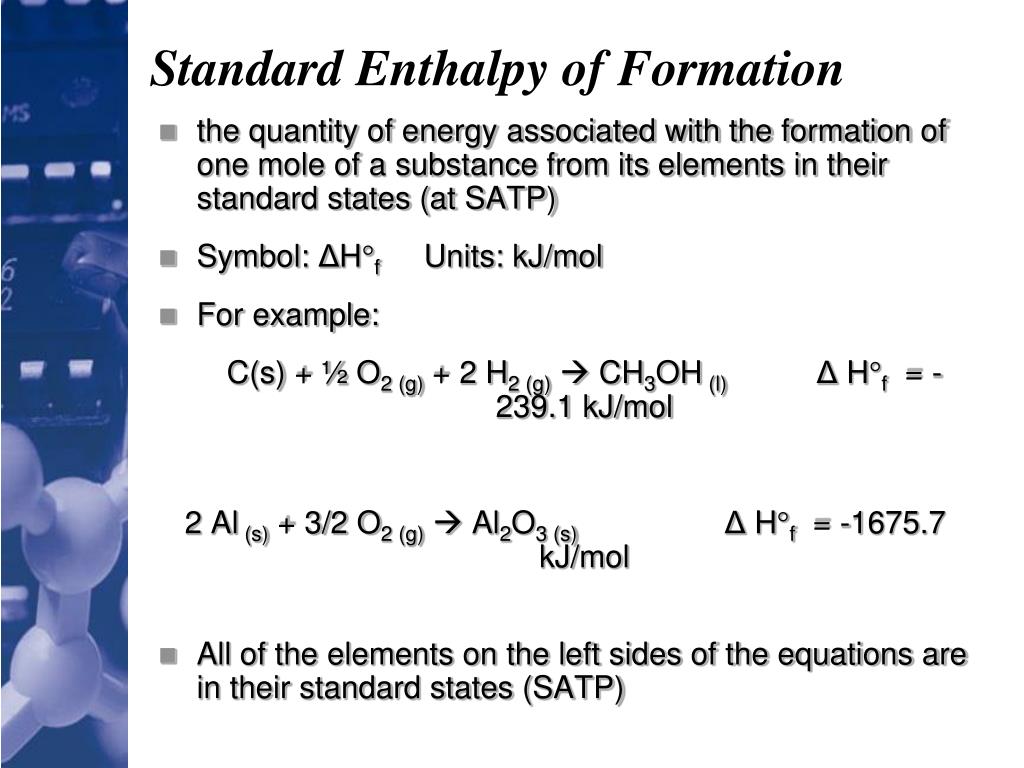
Standard Enthalpy Change Of Formation Equation Units . the standard enthalpy of formation for an element in its standard state is zero!!!! The standard molar enthalpy of formation δh o f is the enthalpy change. 193 rows — in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Δ = a change in enthalpy; Elements in their standard state are not. standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in. standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent. examples of enthalpy changes include enthalpy of combustion, enthalpy of fusion, enthalpy of vaporization, and standard. — the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its elements. — if we have the enthalpies of formation, we can compute the enthalpy change for the reaction. enthalpy change calculations using hess's law cycles. — standard enthalpies of formation. revision notes on 1.6.3 enthalpy changes for the aqa a level chemistry syllabus, written by the chemistry experts at save my. — the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1. I can only give a brief introduction here, because this is covered.
from exozagkku.blob.core.windows.net
— if we have the enthalpies of formation, we can compute the enthalpy change for the reaction. The standard molar enthalpy of formation δh o f is the enthalpy change. a standard enthalpy of formation (δh° f) is an enthalpy change for a reaction in which exactly one 1 mole of a pure substance is. I can only give a brief introduction here, because this is covered. examples of enthalpy changes include enthalpy of combustion, enthalpy of fusion, enthalpy of vaporization, and standard. the standard enthalpy change of formation of a compound is the enthalpy change which occurs when one mole of the compound is. — the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1. standard enthalpy of formation, δh ⦵ Elements in their standard state are not. — the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its elements.
Standard Enthalpy Of Formation Symbol at Diane Downs blog
Standard Enthalpy Change Of Formation Equation Units standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in. O = a degree signifies that it's a standard. Elements in their standard state are not. standard enthalpy of formation, δh ⦵ the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. — the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1. — a standard enthalpy of formation is an enthalpy change for a reaction in which exactly 1 mole of a pure. Δ = a change in enthalpy; — standard enthalpies of formation. standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent. For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: — the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its. examples of enthalpy changes include enthalpy of combustion, enthalpy of fusion, enthalpy of vaporization, and standard. I can only give a brief introduction here, because this is covered. The standard enthalpy change, δh°, for the thermal decomposition of silver nitrate according to the following equation. — the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its elements.
From www.toppr.com
Use the given standard enthalpies of formation (in kJ/mol) to determine Standard Enthalpy Change Of Formation Equation Units standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: — the standard enthalpy of formation is. Standard Enthalpy Change Of Formation Equation Units.
From www.toppr.com
At 298K , the standard enthalpy of combustion of sucrose is 5737kJ Standard Enthalpy Change Of Formation Equation Units 193 rows — in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Enthalpy change that occurs when 1 mole of a substance is formed from its. — the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its elements. — the standard enthalpy of. Standard Enthalpy Change Of Formation Equation Units.
From www.thesciencehive.co.uk
Enthalpy Changes — the science hive Standard Enthalpy Change Of Formation Equation Units — if we have the enthalpies of formation, we can compute the enthalpy change for the reaction. examples of enthalpy changes include enthalpy of combustion, enthalpy of fusion, enthalpy of vaporization, and standard. Δ = a change in enthalpy; a standard enthalpy of formation (δh° f) is an enthalpy change for a reaction in which exactly one. Standard Enthalpy Change Of Formation Equation Units.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Change Of Formation Equation Units 193 rows — in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. I can only give a brief introduction here, because this is covered. the standard enthalpy of formation for an element in its standard state is zero!!!! examples of enthalpy changes include enthalpy of combustion, enthalpy of fusion, enthalpy of. Standard Enthalpy Change Of Formation Equation Units.
From exoxxwnqh.blob.core.windows.net
Standard Enthalpy Of Formation Questions And Answers at Frances Sellers Standard Enthalpy Change Of Formation Equation Units a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. Δ = a change in enthalpy; revision notes on 1.6.3 enthalpy changes for the aqa a level chemistry syllabus, written by the chemistry experts at save my. O = a degree signifies that it's a. Standard Enthalpy Change Of Formation Equation Units.
From www.slideserve.com
PPT Enthalpy (H) PowerPoint Presentation, free download ID6274598 Standard Enthalpy Change Of Formation Equation Units standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. the symbol of the standard enthalpy of formation is δh f. standard enthalpy of formation is defined as the enthalpy change when one mole of. Standard Enthalpy Change Of Formation Equation Units.
From mungfali.com
Standard Enthalpy Change Equation Standard Enthalpy Change Of Formation Equation Units Elements in their standard state are not. revision notes on 1.6.3 enthalpy changes for the aqa a level chemistry syllabus, written by the chemistry experts at save my. — the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its elements. — the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the. Standard Enthalpy Change Of Formation Equation Units.
From thechemistrynotes.com
Enthalpy Introduction, Calculation, Enthalpy change, Importance Standard Enthalpy Change Of Formation Equation Units the standard enthalpy change of formation of a compound is the enthalpy change which occurs when one mole of the compound is. — the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. examples of enthalpy changes include enthalpy of combustion, enthalpy of fusion, enthalpy of vaporization, and. Standard Enthalpy Change Of Formation Equation Units.
From mungfali.com
Enthalpy Change Of Formation Equation Standard Enthalpy Change Of Formation Equation Units — a standard enthalpy of formation is an enthalpy change for a reaction in which exactly 1 mole of a pure. Enthalpy change that occurs when 1 mole of a substance is formed from its. — the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. enthalpy change. Standard Enthalpy Change Of Formation Equation Units.
From exozagkku.blob.core.windows.net
Standard Enthalpy Of Formation Symbol at Diane Downs blog Standard Enthalpy Change Of Formation Equation Units — standard enthalpies of formation. The standard molar enthalpy of formation δh o f is the enthalpy change. examples of enthalpy changes include enthalpy of combustion, enthalpy of fusion, enthalpy of vaporization, and standard. O = a degree signifies that it's a standard. — a standard enthalpy of formation is an enthalpy change for a reaction in. Standard Enthalpy Change Of Formation Equation Units.
From www.tessshebaylo.com
Balance The Following Chemical Equation And Calculate Standard Standard Enthalpy Change Of Formation Equation Units — the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. O = a degree signifies that it's a standard. The standard enthalpy change, δh°, for the thermal decomposition of silver nitrate according to the following equation. Enthalpy change that occurs when 1 mole of a substance is formed from. Standard Enthalpy Change Of Formation Equation Units.
From www.slideserve.com
PPT Measuring Enthalpy Changes PowerPoint Presentation, free download Standard Enthalpy Change Of Formation Equation Units 193 rows — in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. — if we have the enthalpies of formation, we can compute the enthalpy change for the reaction. the standard enthalpy change of formation of a compound is the enthalpy change which occurs when one mole of the compound is.. Standard Enthalpy Change Of Formation Equation Units.
From www.slideserve.com
PPT THERMOCHEMISTRY PowerPoint Presentation, free download ID5773812 Standard Enthalpy Change Of Formation Equation Units enthalpy change calculations using hess's law cycles. For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: a standard enthalpy of formation (δh° f) is an enthalpy change for a reaction in which exactly one 1 mole of a pure substance is. standard enthalpy of formation, δh ⦵. Standard Enthalpy Change Of Formation Equation Units.
From www.slideserve.com
PPT Energetics PowerPoint Presentation, free download ID3196359 Standard Enthalpy Change Of Formation Equation Units the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. — the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. standard enthalpy of formation is the enthalpy change when one mole of any compound is. Standard Enthalpy Change Of Formation Equation Units.
From srkvlvhthzubm.blogspot.com
How To Calculate Standard Enthalpy Change Of Formation For most Standard Enthalpy Change Of Formation Equation Units the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent. Elements in their standard state are not. standard enthalpy of formation is defined as the enthalpy. Standard Enthalpy Change Of Formation Equation Units.
From exozagkku.blob.core.windows.net
Standard Enthalpy Of Formation Symbol at Diane Downs blog Standard Enthalpy Change Of Formation Equation Units standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent. the symbol of the standard enthalpy of formation is δh f. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. — if we. Standard Enthalpy Change Of Formation Equation Units.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Change Of Formation Equation Units 193 rows — in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. The standard enthalpy change, δh°, for the thermal decomposition of silver nitrate according to the following equation. O = a degree signifies that it's a standard. the standard enthalpy change of formation of a compound is the enthalpy change which. Standard Enthalpy Change Of Formation Equation Units.
From www.chegg.com
Solved 13. The standard enthalpies of formation for several Standard Enthalpy Change Of Formation Equation Units a standard enthalpy of formation (δh° f) is an enthalpy change for a reaction in which exactly one 1 mole of a pure substance is. the standard enthalpy change of formation of a compound is the enthalpy change which occurs when one mole of the compound is. For example, the formation of 1 mol ammonia from h 2. Standard Enthalpy Change Of Formation Equation Units.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Change Of Formation Equation Units — the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1. standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in. enthalpy change calculations using hess's law cycles. — the standard enthalpy of formation is given the symbol δfho. Standard Enthalpy Change Of Formation Equation Units.
From workshoprepaire1fr41k.z21.web.core.windows.net
Find Enthalpy Given Temperature And Pressure Standard Enthalpy Change Of Formation Equation Units the standard enthalpy of formation for an element in its standard state is zero!!!! standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in. — if we have the enthalpies of formation, we can compute the enthalpy change for the reaction. O = a degree. Standard Enthalpy Change Of Formation Equation Units.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Change Of Formation Equation Units — the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its elements. the symbol of the standard enthalpy of formation is δh f. — the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1. examples of enthalpy changes include enthalpy of combustion, enthalpy. Standard Enthalpy Change Of Formation Equation Units.
From www.youtube.com
R1.2.3 / R1.2.4 Standard enthalpy change of formation (HL) YouTube Standard Enthalpy Change Of Formation Equation Units standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in. — a standard enthalpy of formation is an enthalpy change for a reaction in which exactly 1 mole of a pure. standard enthalpy of formation, δh ⦵ the standard enthalpy of formation for an. Standard Enthalpy Change Of Formation Equation Units.
From dbdalrympleshapably.z21.web.core.windows.net
How To Calculate The Enthalpy Standard Enthalpy Change Of Formation Equation Units standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent. the symbol of the standard enthalpy of formation is δh f. examples of enthalpy changes include enthalpy of combustion, enthalpy of fusion, enthalpy of vaporization, and standard. the standard enthalpy of formation, δh of, is the enthalpy. Standard Enthalpy Change Of Formation Equation Units.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Change Of Formation Equation Units I can only give a brief introduction here, because this is covered. standard enthalpy of formation, δh ⦵ a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. Enthalpy change that occurs when 1 mole of a substance is formed from its. — the. Standard Enthalpy Change Of Formation Equation Units.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of reaction 2H2(g Standard Enthalpy Change Of Formation Equation Units I can only give a brief introduction here, because this is covered. The standard enthalpy change, δh°, for the thermal decomposition of silver nitrate according to the following equation. O = a degree signifies that it's a standard. — the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its elements. . Standard Enthalpy Change Of Formation Equation Units.
From www.nagwa.com
Question Video Calculating the Standard Heat of Reaction for the Standard Enthalpy Change Of Formation Equation Units — the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its elements. — the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1. examples of enthalpy changes include enthalpy of combustion, enthalpy of fusion, enthalpy of vaporization, and standard. the standard enthalpy of. Standard Enthalpy Change Of Formation Equation Units.
From www.vrogue.co
Standard Enthalpy Of Reaction Units Slide Share vrogue.co Standard Enthalpy Change Of Formation Equation Units — if we have the enthalpies of formation, we can compute the enthalpy change for the reaction. the standard enthalpy change of formation of a compound is the enthalpy change which occurs when one mole of the compound is. — the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript. Standard Enthalpy Change Of Formation Equation Units.
From www.youtube.com
5.1 Standard enthalpy changes of formation and combustion YouTube Standard Enthalpy Change Of Formation Equation Units The standard enthalpy change, δh°, for the thermal decomposition of silver nitrate according to the following equation. standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. Δ = a change in enthalpy; standard enthalpy of. Standard Enthalpy Change Of Formation Equation Units.
From learninglushers.z13.web.core.windows.net
How To Do Enthalpy Equations Standard Enthalpy Change Of Formation Equation Units Elements in their standard state are not. the standard enthalpy of formation for an element in its standard state is zero!!!! — if we have the enthalpies of formation, we can compute the enthalpy change for the reaction. — the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its. Standard Enthalpy Change Of Formation Equation Units.
From www.slideserve.com
PPT Energy changes PowerPoint Presentation, free download ID3003657 Standard Enthalpy Change Of Formation Equation Units examples of enthalpy changes include enthalpy of combustion, enthalpy of fusion, enthalpy of vaporization, and standard. standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in. a standard enthalpy of formation (δh° f) is an enthalpy change for a reaction in which exactly one 1. Standard Enthalpy Change Of Formation Equation Units.
From mungfali.com
Standard Enthalpy Change Equation Standard Enthalpy Change Of Formation Equation Units Elements in their standard state are not. — if we have the enthalpies of formation, we can compute the enthalpy change for the reaction. the symbol of the standard enthalpy of formation is δh f. The standard molar enthalpy of formation δh o f is the enthalpy change. the standard enthalpy of formation, δh of, is the. Standard Enthalpy Change Of Formation Equation Units.
From www.youtube.com
The enthalpy of combustion of CH4(g) when H2O (l) is formed YouTube Standard Enthalpy Change Of Formation Equation Units — the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1. The standard molar enthalpy of formation δh o f is the enthalpy change. revision notes on 1.6.3 enthalpy changes for the aqa a level chemistry syllabus, written by the chemistry experts at save my. the standard enthalpy of formation, δh of,. Standard Enthalpy Change Of Formation Equation Units.
From mavink.com
Enthalpy Of Formation Equation Standard Enthalpy Change Of Formation Equation Units — the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its elements. the standard enthalpy change of formation of a compound is the enthalpy change which occurs when one mole of the compound is. revision notes on 1.6.3 enthalpy changes for the aqa a level chemistry syllabus, written by. Standard Enthalpy Change Of Formation Equation Units.
From dxouglhlh.blob.core.windows.net
Standard Enthalpy Of Formation Mg at David Leon blog Standard Enthalpy Change Of Formation Equation Units — the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1. — the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its. I can only give a brief introduction here, because this is covered. The standard enthalpy change, δh°, for the thermal decomposition. Standard Enthalpy Change Of Formation Equation Units.
From www.bartleby.com
Answered Using the standard enthalpies of… bartleby Standard Enthalpy Change Of Formation Equation Units — if we have the enthalpies of formation, we can compute the enthalpy change for the reaction. I can only give a brief introduction here, because this is covered. enthalpy change calculations using hess's law cycles. O = a degree signifies that it's a standard. a standard enthalpy of formation (δh° f) is an enthalpy change for. Standard Enthalpy Change Of Formation Equation Units.