What Is Dilution In Chemistry Class 10 . explain how concentrations can be changed in the lab. Understand how stock solutions are used in the laboratory. The chemist can do it simply by mixing with more. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. dilution is the process of reducing the concentration of a given solute in its solution. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Concentration is the removal of solvent, which increases the concentration. dilution is the addition of solvent, which decreases the concentration of the solute in the solution.
from cejakhtq.blob.core.windows.net
dilution is the process of reducing the concentration of a given solute in its solution. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. The chemist can do it simply by mixing with more. Concentration is the removal of solvent, which increases the concentration. explain how concentrations can be changed in the lab. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. Understand how stock solutions are used in the laboratory. dilution is the addition of solvent, which decreases the concentration of the solute in the solution.
How To Do A Dilution Experiment at Lillian Benda blog
What Is Dilution In Chemistry Class 10 explain how concentrations can be changed in the lab. The chemist can do it simply by mixing with more. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Understand how stock solutions are used in the laboratory. dilution is the process of reducing the concentration of a given solute in its solution. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. explain how concentrations can be changed in the lab.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query What Is Dilution In Chemistry Class 10 dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. explain how concentrations can be changed in the lab. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution. What Is Dilution In Chemistry Class 10.
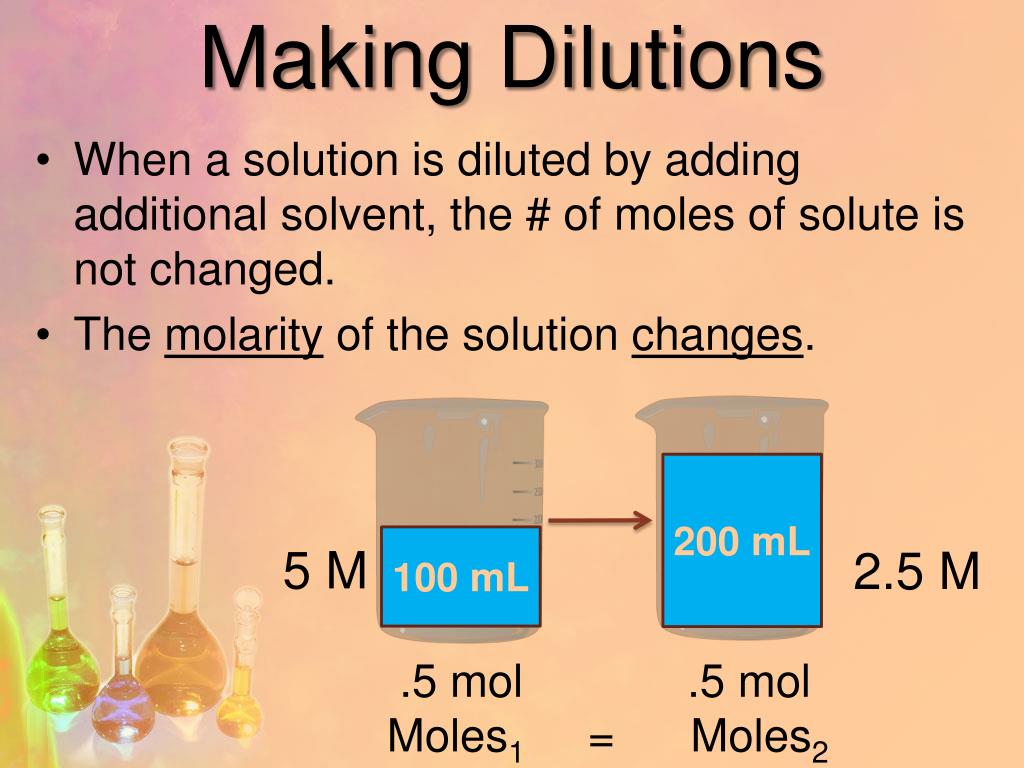
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID5812279 What Is Dilution In Chemistry Class 10 explain how concentrations can be changed in the lab. Understand how stock solutions are used in the laboratory. The chemist can do it simply by mixing with more. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. dilution is the process of reducing the concentration of. What Is Dilution In Chemistry Class 10.
From www.youtube.com
Dilution problems Chemistry Molarity & Concentration Examples What Is Dilution In Chemistry Class 10 explain how concentrations can be changed in the lab. The chemist can do it simply by mixing with more. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Understand how stock solutions are used in the laboratory. Concentration is the removal of solvent, which increases the concentration. the. What Is Dilution In Chemistry Class 10.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate What Is Dilution In Chemistry Class 10 the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. explain how concentrations can be changed in the lab. Understand how stock solutions are used in the laboratory. The. What Is Dilution In Chemistry Class 10.
From materialschoolpinder.z21.web.core.windows.net
How To Do Dilutions In Chemistry What Is Dilution In Chemistry Class 10 Understand how stock solutions are used in the laboratory. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. dilution is the process of reducing the concentration of a given solute. What Is Dilution In Chemistry Class 10.
From teachsimple.com
Preparing Solutions and Dilutions Senior Chemistry lesson by Teach Simple What Is Dilution In Chemistry Class 10 Understand how stock solutions are used in the laboratory. The chemist can do it simply by mixing with more. Concentration is the removal of solvent, which increases the concentration. dilution is the process of reducing the concentration of a given solute in its solution. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1). What Is Dilution In Chemistry Class 10.
From cejioehy.blob.core.windows.net
Dilute Meaning In Chemistry at Benjamin Hack blog What Is Dilution In Chemistry Class 10 dilution is the process of reducing the concentration of a given solute in its solution. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Understand how stock solutions are used in the laboratory. Concentration is the removal of solvent, which increases the concentration. the dilution formula (c1v1 =. What Is Dilution In Chemistry Class 10.
From www.ck12.org
Dilution (M[i]V[i]=M[f]V[f]) Example 1 ( Video ) Chemistry CK12 What Is Dilution In Chemistry Class 10 Understand how stock solutions are used in the laboratory. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. dilution is the addition of solvent, which decreases the concentration. What Is Dilution In Chemistry Class 10.
From cexkiyfv.blob.core.windows.net
How To Do Dilutions In Chemistry at Gussie Jasmin blog What Is Dilution In Chemistry Class 10 The chemist can do it simply by mixing with more. dilution is the process of reducing the concentration of a given solute in its solution. Understand how stock solutions are used in the laboratory. Concentration is the removal of solvent, which increases the concentration. dilution is the addition of solvent, which decreases the concentration of the solute in. What Is Dilution In Chemistry Class 10.
From cexkiyfv.blob.core.windows.net
How To Do Dilutions In Chemistry at Gussie Jasmin blog What Is Dilution In Chemistry Class 10 dilution is the process of reducing the concentration of a given solute in its solution. Understand how stock solutions are used in the laboratory. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. Concentration is the removal of solvent, which increases the concentration. The chemist can do. What Is Dilution In Chemistry Class 10.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii What Is Dilution In Chemistry Class 10 Understand how stock solutions are used in the laboratory. The chemist can do it simply by mixing with more. dilution is the process of reducing the concentration of a given solute in its solution. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. explain how concentrations can be. What Is Dilution In Chemistry Class 10.
From www.slideshare.net
Chapter 16 solutions What Is Dilution In Chemistry Class 10 the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Understand how stock solutions are used in the laboratory. explain how concentrations can be changed in the lab. Concentration is the removal of. What Is Dilution In Chemistry Class 10.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube What Is Dilution In Chemistry Class 10 The chemist can do it simply by mixing with more. dilution is the process of reducing the concentration of a given solute in its solution. explain how concentrations can be changed in the lab. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Understand how stock solutions are. What Is Dilution In Chemistry Class 10.
From www.youtube.com
CBSE Class 10 Chemistry Notes Dilution YouTube What Is Dilution In Chemistry Class 10 Concentration is the removal of solvent, which increases the concentration. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the process of reducing the concentration of a given solute in its solution. explain how concentrations can be changed in the lab. The chemist can do it simply by. What Is Dilution In Chemistry Class 10.
From cejakhtq.blob.core.windows.net
How To Do A Dilution Experiment at Lillian Benda blog What Is Dilution In Chemistry Class 10 dilution is the process of reducing the concentration of a given solute in its solution. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Understand how stock solutions are used in the laboratory. Concentration is the removal of solvent, which increases the concentration. dilution is the addition of. What Is Dilution In Chemistry Class 10.
From ar.inspiredpencil.com
Dilute Science What Is Dilution In Chemistry Class 10 the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. Concentration is the removal of solvent, which increases the concentration. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Understand how stock solutions are used in the laboratory. dilution is. What Is Dilution In Chemistry Class 10.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download What Is Dilution In Chemistry Class 10 dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Understand how stock solutions are used in the laboratory. Concentration is the removal of solvent, which increases the concentration. explain how concentrations can be changed in the lab. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution. What Is Dilution In Chemistry Class 10.
From www.studocu.com
Copy of Dilutions in Chemistry Dilutions In the example above kool What Is Dilution In Chemistry Class 10 dilution is the process of reducing the concentration of a given solute in its solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. the dilution formula (c1v1 = c2v2) calculates. What Is Dilution In Chemistry Class 10.
From www.pinterest.cl
Dilution when solvent is added to dilute a solution, the number of What Is Dilution In Chemistry Class 10 dilution is the process of reducing the concentration of a given solute in its solution. explain how concentrations can be changed in the lab. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. Concentration is the removal of solvent, which increases the concentration. the dilution. What Is Dilution In Chemistry Class 10.
From www.studypool.com
SOLUTION Dilution of solution from chemistry for class 10th Studypool What Is Dilution In Chemistry Class 10 Concentration is the removal of solvent, which increases the concentration. explain how concentrations can be changed in the lab. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the process of reducing the concentration of a given solute in its solution. The chemist can do it simply by. What Is Dilution In Chemistry Class 10.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts What Is Dilution In Chemistry Class 10 the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Concentration is the removal of solvent, which increases the concentration. explain how concentrations can be changed in the lab. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a.. What Is Dilution In Chemistry Class 10.
From www.youtube.com
ACIDS, BASES AND SALTS 04 Dilution of An Acid Chemistry Class What Is Dilution In Chemistry Class 10 Understand how stock solutions are used in the laboratory. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the process of reducing the concentration of a given solute in its solution. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent. What Is Dilution In Chemistry Class 10.
From www.youtube.com
Dilution of Concentrated Acid Chapter Acids, Bases, Salts What Is Dilution In Chemistry Class 10 The chemist can do it simply by mixing with more. Understand how stock solutions are used in the laboratory. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. the dilution formula (c1v1. What Is Dilution In Chemistry Class 10.
From www.youtube.com
Concentrated/dilute acid Dilution of acid Basicity of acid 10th What Is Dilution In Chemistry Class 10 dilution is the process of reducing the concentration of a given solute in its solution. Understand how stock solutions are used in the laboratory. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. explain how concentrations can be changed in the lab. Concentration is the removal. What Is Dilution In Chemistry Class 10.
From www.youtube.com
What Is Dilution? Chemistry Matters YouTube What Is Dilution In Chemistry Class 10 dilution is the process of reducing the concentration of a given solute in its solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Understand how stock solutions are used in the laboratory. the dilution equation is a simple relation between concentrations and volumes of a solution before and after. What Is Dilution In Chemistry Class 10.
From zhtutorials.com
Serial Dilution Practical Skills Ep 3 Zoë Huggett Tutorials What Is Dilution In Chemistry Class 10 explain how concentrations can be changed in the lab. The chemist can do it simply by mixing with more. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Concentration is the removal. What Is Dilution In Chemistry Class 10.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript What Is Dilution In Chemistry Class 10 the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Understand how stock solutions are used in the laboratory. dilution is the process of reducing the concentration of a given solute in its solution. Concentration is the removal of solvent, which increases the concentration. explain how concentrations can be. What Is Dilution In Chemistry Class 10.
From www.youtube.com
10th Chemistry I Are All the Compounds Having Hydrogen acids? I What Is Dilution In Chemistry Class 10 the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the. What Is Dilution In Chemistry Class 10.
From www.w3schools.blog
Standard enthalpy of dilution W3schools What Is Dilution In Chemistry Class 10 dilution is the process of reducing the concentration of a given solute in its solution. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. Concentration is the removal of solvent, which increases the concentration. Understand how stock solutions are used in the laboratory. explain how concentrations. What Is Dilution In Chemistry Class 10.
From materialschoolpinder.z21.web.core.windows.net
How To Do Dilutions In Chemistry What Is Dilution In Chemistry Class 10 dilution is the process of reducing the concentration of a given solute in its solution. Concentration is the removal of solvent, which increases the concentration. Understand how stock solutions are used in the laboratory. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. explain how concentrations. What Is Dilution In Chemistry Class 10.
From materialschoolpinder.z21.web.core.windows.net
How To Do Dilutions In Chemistry What Is Dilution In Chemistry Class 10 dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the process of reducing the concentration of a given solute in its solution. explain how concentrations can be changed in the lab. The chemist can do it simply by mixing with more. Understand how stock solutions are used in. What Is Dilution In Chemistry Class 10.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube What Is Dilution In Chemistry Class 10 the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Understand how stock solutions are used in the laboratory. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. explain how concentrations can be changed in the lab. . What Is Dilution In Chemistry Class 10.
From materialschoolpinder.z21.web.core.windows.net
How To Do Dilutions In Chemistry What Is Dilution In Chemistry Class 10 The chemist can do it simply by mixing with more. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. explain how concentrations can be changed in the lab. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution.. What Is Dilution In Chemistry Class 10.
From carlosgokeowen.blogspot.com
What is Dilution What Is Dilution In Chemistry Class 10 Concentration is the removal of solvent, which increases the concentration. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. dilution is the process of reducing the concentration of a given. What Is Dilution In Chemistry Class 10.
From www.youtube.com
Beginning Chemistry Solution Dilution YouTube What Is Dilution In Chemistry Class 10 the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. The chemist can do it simply by mixing with more. Understand how stock solutions are used in the laboratory. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. . What Is Dilution In Chemistry Class 10.