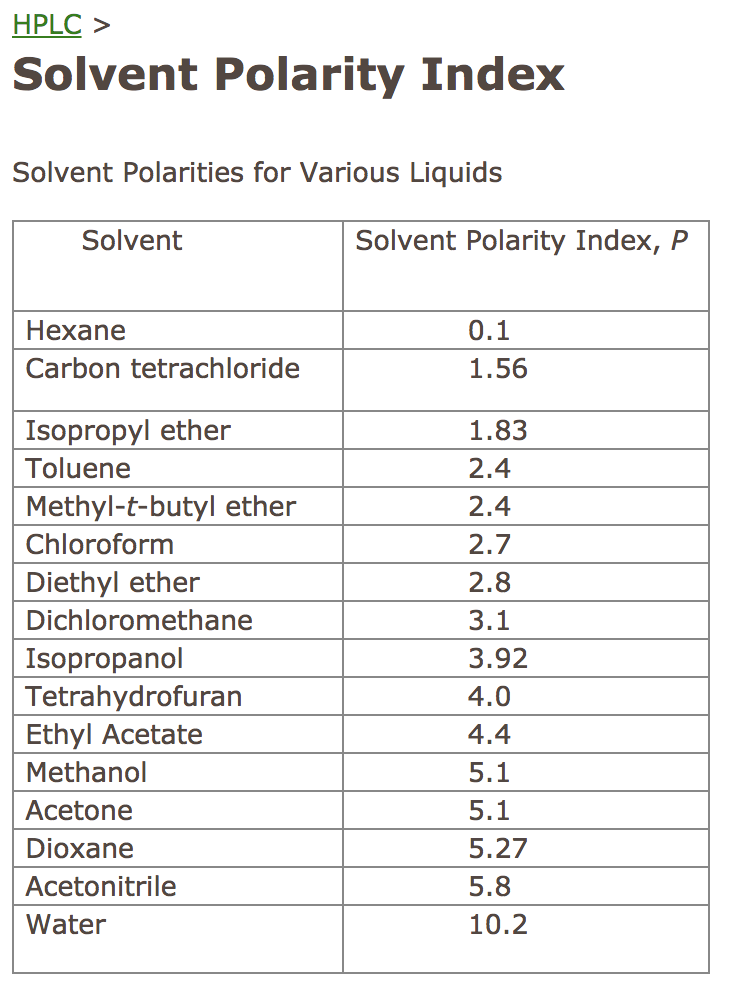
Organic Solvent Relative Polarity . Common solvents arranged from the least polar to the most polar Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. The simple understanding of polarizability is how easily the electrons get. The relative polarizability of electrons. Journal of the american chemical society. Empirical solvent polarity parameters of organic. The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were. Virtually all of the organic chemistry that you will see in this course takes place in the solution phase. Solvents used in organic chemistry are characterized by their. 23 rows solvent polarity;
from www.chegg.com
Solvents used in organic chemistry are characterized by their. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. The relative polarizability of electrons. Common solvents arranged from the least polar to the most polar Virtually all of the organic chemistry that you will see in this course takes place in the solution phase. The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were. Empirical solvent polarity parameters of organic. 23 rows solvent polarity; The simple understanding of polarizability is how easily the electrons get. Journal of the american chemical society.
Solved Determine the solvent polarity index for the
Organic Solvent Relative Polarity The relative polarizability of electrons. The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were. Virtually all of the organic chemistry that you will see in this course takes place in the solution phase. The simple understanding of polarizability is how easily the electrons get. Solvents used in organic chemistry are characterized by their. The relative polarizability of electrons. Common solvents arranged from the least polar to the most polar 23 rows solvent polarity; Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Empirical solvent polarity parameters of organic. Journal of the american chemical society.
From techblog.ctgclean.com
Chemistry Solvent Characteristics CTG Clean Organic Solvent Relative Polarity Common solvents arranged from the least polar to the most polar The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were. Virtually all of the organic chemistry that you will see in this course takes place in the solution phase. The relative polarizability of electrons. Solvents used in organic chemistry are characterized by. Organic Solvent Relative Polarity.
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Organic Solvent Relative Polarity 23 rows solvent polarity; Virtually all of the organic chemistry that you will see in this course takes place in the solution phase. Empirical solvent polarity parameters of organic. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were.. Organic Solvent Relative Polarity.
From www.researchgate.net
Comparison of the Polarity Index of Organic Solvents Download Organic Solvent Relative Polarity The simple understanding of polarizability is how easily the electrons get. The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Common solvents arranged from the least polar to the most polar Empirical solvent polarity parameters of organic. 23. Organic Solvent Relative Polarity.
From www.researchgate.net
Comparison of the Polarity Index of Organic Solvents Download Organic Solvent Relative Polarity Empirical solvent polarity parameters of organic. 23 rows solvent polarity; Solvents used in organic chemistry are characterized by their. Journal of the american chemical society. The relative polarizability of electrons. The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide. Organic Solvent Relative Polarity.
From www.chegg.com
Solved Match the following organic solvents (structures Organic Solvent Relative Polarity The simple understanding of polarizability is how easily the electrons get. The relative polarizability of electrons. Virtually all of the organic chemistry that you will see in this course takes place in the solution phase. Empirical solvent polarity parameters of organic. Journal of the american chemical society. Common solvents arranged from the least polar to the most polar Water acetic. Organic Solvent Relative Polarity.
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Organic Solvent Relative Polarity Virtually all of the organic chemistry that you will see in this course takes place in the solution phase. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. 23 rows solvent polarity; The simple understanding of polarizability is how easily the electrons get. Solvents used in organic chemistry are characterized by their. Empirical solvent polarity. Organic Solvent Relative Polarity.
From www.chegg.com
Solved Determine the solvent polarity index for the Organic Solvent Relative Polarity Common solvents arranged from the least polar to the most polar Journal of the american chemical society. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Empirical solvent polarity parameters of organic. 23 rows solvent polarity; The relative polarizability of electrons. Virtually all of the organic chemistry that you will see in this course takes. Organic Solvent Relative Polarity.
From www.pinterest.com
the effect of polar and aprotic solutens on nucleophilia and basicity Organic Solvent Relative Polarity The simple understanding of polarizability is how easily the electrons get. 23 rows solvent polarity; Empirical solvent polarity parameters of organic. The relative polarizability of electrons. Solvents used in organic chemistry are characterized by their. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Virtually all of the organic chemistry that you will see in. Organic Solvent Relative Polarity.
From mavink.com
Polarity Chart Of Solvents Organic Solvent Relative Polarity The simple understanding of polarizability is how easily the electrons get. The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were. Solvents used in organic chemistry are characterized by their. Empirical solvent polarity parameters of organic. 23 rows solvent polarity; Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide. Organic Solvent Relative Polarity.
From aceorganicchem.com
Organic Chem 15 For organic solvents, likes dissolve likes Organic Solvent Relative Polarity The simple understanding of polarizability is how easily the electrons get. Solvents used in organic chemistry are characterized by their. Journal of the american chemical society. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were. The relative polarizability. Organic Solvent Relative Polarity.
From studylib.net
Common Organic Solvents by PolarityF22 Organic Solvent Relative Polarity Empirical solvent polarity parameters of organic. 23 rows solvent polarity; The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were. Solvents used in organic chemistry are characterized by their. Journal of the american chemical society. Virtually all of the organic chemistry that you will see in this course takes place in the solution. Organic Solvent Relative Polarity.
From www.researchgate.net
Organic solvent classification according to the degree of polarity by Organic Solvent Relative Polarity Empirical solvent polarity parameters of organic. Solvents used in organic chemistry are characterized by their. Common solvents arranged from the least polar to the most polar The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were. The relative polarizability of electrons. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline. Organic Solvent Relative Polarity.
From bceweb.org
Organic Solvent Polarity Chart A Visual Reference of Charts Chart Master Organic Solvent Relative Polarity Solvents used in organic chemistry are characterized by their. Virtually all of the organic chemistry that you will see in this course takes place in the solution phase. 23 rows solvent polarity; Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. The relative polarizability of electrons. Journal of the american chemical society. The simple understanding. Organic Solvent Relative Polarity.
From www.semanticscholar.org
Table 1 from Classification of organic solvents based on correlation Organic Solvent Relative Polarity Solvents used in organic chemistry are characterized by their. 23 rows solvent polarity; Virtually all of the organic chemistry that you will see in this course takes place in the solution phase. The simple understanding of polarizability is how easily the electrons get. Common solvents arranged from the least polar to the most polar The relative polarizability of electrons. Journal. Organic Solvent Relative Polarity.
From www.researchgate.net
The Polarity Indices (P') and the Selectivity Parameters (x e , x d Organic Solvent Relative Polarity Virtually all of the organic chemistry that you will see in this course takes place in the solution phase. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. 23 rows solvent polarity; Journal of the american chemical society. Empirical solvent polarity parameters of organic. Solvents used in organic chemistry are characterized by their. Common solvents. Organic Solvent Relative Polarity.
From www.researchgate.net
Physical properties of some common organic solvents. Solvent Formula Organic Solvent Relative Polarity The relative polarizability of electrons. The simple understanding of polarizability is how easily the electrons get. Solvents used in organic chemistry are characterized by their. Virtually all of the organic chemistry that you will see in this course takes place in the solution phase. Journal of the american chemical society. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Organic Solvent Relative Polarity.
From alexmistry.z13.web.core.windows.net
Polarity Of Organic Solvents Chart Organic Solvent Relative Polarity Virtually all of the organic chemistry that you will see in this course takes place in the solution phase. Empirical solvent polarity parameters of organic. The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were. The relative polarizability of electrons. The simple understanding of polarizability is how easily the electrons get. Water acetic. Organic Solvent Relative Polarity.
From bceweb.org
Polarity Chart Of Organic Solvents A Visual Reference of Charts Organic Solvent Relative Polarity Journal of the american chemical society. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. The relative polarizability of electrons. The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were. 23 rows solvent polarity; Solvents used in organic chemistry are characterized by their. Virtually all of the organic. Organic Solvent Relative Polarity.
From www.wou.edu
CH105 Chapter 9 Organic Compounds of Oxygen Chemistry Organic Solvent Relative Polarity Virtually all of the organic chemistry that you will see in this course takes place in the solution phase. 23 rows solvent polarity; Journal of the american chemical society. The simple understanding of polarizability is how easily the electrons get. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. The relative polarizability of electrons. Empirical. Organic Solvent Relative Polarity.
From mungfali.com
Solvent Polarity Chart Organic Solvent Relative Polarity The relative polarizability of electrons. Common solvents arranged from the least polar to the most polar Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Virtually all of the organic chemistry that you will see in this course takes place in the solution phase. The simple understanding of polarizability is how easily the electrons get.. Organic Solvent Relative Polarity.
From www.researchgate.net
Nature of solvents, its polarity and general uses Download Scientific Organic Solvent Relative Polarity 23 rows solvent polarity; The relative polarizability of electrons. Common solvents arranged from the least polar to the most polar Journal of the american chemical society. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were. Virtually all of. Organic Solvent Relative Polarity.
From www.researchgate.net
Common organic solvents' structures [11]. Download Scientific Diagram Organic Solvent Relative Polarity The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were. Journal of the american chemical society. The simple understanding of polarizability is how easily the electrons get. The relative polarizability of electrons. Empirical solvent polarity parameters of organic. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Solvents. Organic Solvent Relative Polarity.
From mungfali.com
Solvent Polarity Chart Organic Solvent Relative Polarity The relative polarizability of electrons. Solvents used in organic chemistry are characterized by their. 23 rows solvent polarity; Virtually all of the organic chemistry that you will see in this course takes place in the solution phase. Empirical solvent polarity parameters of organic. The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were.. Organic Solvent Relative Polarity.
From www.researchgate.net
Relative solvent polarity E T (&) and 2DG CH 2 () for selected Organic Solvent Relative Polarity Common solvents arranged from the least polar to the most polar The relative polarizability of electrons. Solvents used in organic chemistry are characterized by their. 23 rows solvent polarity; Empirical solvent polarity parameters of organic. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. The values for relative polarity are normalized from measurements of solvent. Organic Solvent Relative Polarity.
From www.masterorganicchemistry.com
All about Solvents NonPolar, Polar Aprotic, and Polar Protic Solvents Organic Solvent Relative Polarity Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Empirical solvent polarity parameters of organic. Virtually all of the organic chemistry that you will see in this course takes place in the solution phase. Solvents used in organic chemistry are characterized by their. The relative polarizability of electrons. Journal of the american chemical society. 23. Organic Solvent Relative Polarity.
From bamil.lewisburgdistrictumc.org
organic solvent polarity chart Bamil Organic Solvent Relative Polarity The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were. Common solvents arranged from the least polar to the most polar The relative polarizability of electrons. 23 rows solvent polarity; Journal of the american chemical society. The simple understanding of polarizability is how easily the electrons get. Empirical solvent polarity parameters of organic.. Organic Solvent Relative Polarity.
From www.coursehero.com
[Solved] . Solvent Boiling Point ( 'C) Relative Polarity Solvent Front Organic Solvent Relative Polarity Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. The simple understanding of polarizability is how easily the electrons get. 23 rows solvent polarity; Virtually all of the organic chemistry that you will see in this course takes place in the solution phase. Solvents used in organic chemistry are characterized by their. Common solvents arranged. Organic Solvent Relative Polarity.
From mungfali.com
Polarity Index Chart Organic Solvent Relative Polarity Solvents used in organic chemistry are characterized by their. Common solvents arranged from the least polar to the most polar The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were. Virtually all of the organic chemistry that you will see in this course takes place in the solution phase. The relative polarizability of. Organic Solvent Relative Polarity.
From www.chegg.com
Solved Determine the solvent polarity index for each HPLC Organic Solvent Relative Polarity Solvents used in organic chemistry are characterized by their. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. The relative polarizability of electrons. Journal of the american chemical society. 23 rows solvent polarity; The simple understanding of polarizability is how easily the electrons get. Common solvents arranged from the least polar to the most polar. Organic Solvent Relative Polarity.
From www.scribd.com
Polarity of Solvents PDF Organic Solvent Relative Polarity Virtually all of the organic chemistry that you will see in this course takes place in the solution phase. The relative polarizability of electrons. 23 rows solvent polarity; Solvents used in organic chemistry are characterized by their. The simple understanding of polarizability is how easily the electrons get. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline. Organic Solvent Relative Polarity.
From www.chegg.com
Solved Match the following organic solvents (structures Organic Solvent Relative Polarity The relative polarizability of electrons. Virtually all of the organic chemistry that you will see in this course takes place in the solution phase. The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were. 23 rows solvent polarity; The simple understanding of polarizability is how easily the electrons get. Empirical solvent polarity parameters. Organic Solvent Relative Polarity.
From mavink.com
Functional Group Polarity Chart Organic Solvent Relative Polarity Virtually all of the organic chemistry that you will see in this course takes place in the solution phase. The relative polarizability of electrons. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Journal of the american chemical society. The simple understanding of polarizability is how easily the electrons get. The values for relative polarity. Organic Solvent Relative Polarity.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID4409461 Organic Solvent Relative Polarity The simple understanding of polarizability is how easily the electrons get. The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were. Common solvents arranged from the least polar to the most polar Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Solvents used in organic chemistry are characterized. Organic Solvent Relative Polarity.
From chartdata.web.app
Polarity Chart Of Solvents Organic Solvent Relative Polarity Virtually all of the organic chemistry that you will see in this course takes place in the solution phase. Common solvents arranged from the least polar to the most polar The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were. The relative polarizability of electrons. Empirical solvent polarity parameters of organic. Journal of. Organic Solvent Relative Polarity.
From www.researchgate.net
Solvent polarity and eluotropic strength (ɛ°) Download Scientific Diagram Organic Solvent Relative Polarity Virtually all of the organic chemistry that you will see in this course takes place in the solution phase. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. The simple understanding of polarizability is how easily the electrons get. The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and. Organic Solvent Relative Polarity.