Bromide Ion Silver . silver bromide (agbr) is a chemical compound of silver and bromine. See the reactions, products, solubility products and. Adding iron(ii) solution to the silver. silver bromide is a pale yellow crystalline solid with the formula agbr. Learn how to prepare it, its. silver chloride dissolves readily in ammonia solution, while the bromide partially dissolves and the iodide does not. It has a rock salt structure and is light sensitive. learn how to identify halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. Learn more about its properties,. It has a molecular weight of 187.772 and a cas. silver bromide is a pale yellow compound, which upon exposure to light, darkens due to the photochemical decomposition that.
from www.numerade.com
Learn how to prepare it, its. It has a rock salt structure and is light sensitive. silver bromide (agbr) is a chemical compound of silver and bromine. silver bromide is a pale yellow crystalline solid with the formula agbr. See the reactions, products, solubility products and. silver chloride dissolves readily in ammonia solution, while the bromide partially dissolves and the iodide does not. silver bromide is a pale yellow compound, which upon exposure to light, darkens due to the photochemical decomposition that. Learn more about its properties,. learn how to identify halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. It has a molecular weight of 187.772 and a cas.
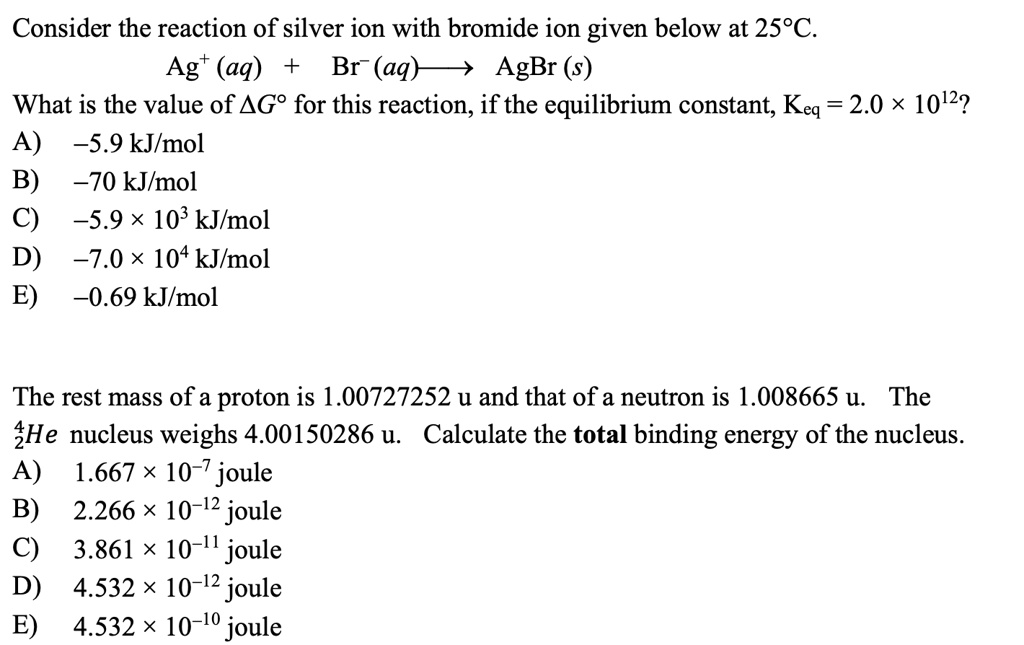
SOLVED Consider the reaction of silver ion with bromide ion given
Bromide Ion Silver silver chloride dissolves readily in ammonia solution, while the bromide partially dissolves and the iodide does not. It has a molecular weight of 187.772 and a cas. Learn more about its properties,. Adding iron(ii) solution to the silver. See the reactions, products, solubility products and. silver bromide (agbr) is a chemical compound of silver and bromine. learn how to identify halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. It has a rock salt structure and is light sensitive. silver chloride dissolves readily in ammonia solution, while the bromide partially dissolves and the iodide does not. Learn how to prepare it, its. silver bromide is a pale yellow crystalline solid with the formula agbr. silver bromide is a pale yellow compound, which upon exposure to light, darkens due to the photochemical decomposition that.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube Bromide Ion Silver silver chloride dissolves readily in ammonia solution, while the bromide partially dissolves and the iodide does not. silver bromide is a pale yellow crystalline solid with the formula agbr. Learn how to prepare it, its. Learn more about its properties,. silver bromide (agbr) is a chemical compound of silver and bromine. See the reactions, products, solubility products. Bromide Ion Silver.
From www.t3db.ca
T3DB Silver bromide Bromide Ion Silver See the reactions, products, solubility products and. Adding iron(ii) solution to the silver. learn how to identify halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. Learn more about its properties,. silver bromide (agbr) is a chemical compound of silver and bromine. silver bromide is a pale yellow crystalline solid with the formula agbr.. Bromide Ion Silver.
From dxodjrzxh.blob.core.windows.net
Bromide Ion Experiment at Frank Radcliff blog Bromide Ion Silver See the reactions, products, solubility products and. learn how to identify halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. silver bromide (agbr) is a chemical compound of silver and bromine. Learn how to prepare it, its. silver bromide is a pale yellow crystalline solid with the formula agbr. It has a rock salt. Bromide Ion Silver.
From www.nagwa.com
Question Video Determining Which Ions Are Oxidized and Reduced in the Bromide Ion Silver learn how to identify halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. silver bromide (agbr) is a chemical compound of silver and bromine. Learn more about its properties,. Adding iron(ii) solution to the silver. See the reactions, products, solubility products and. Learn how to prepare it, its. silver chloride dissolves readily in ammonia. Bromide Ion Silver.
From www.youtube.com
How to identify chloride, bromide and iodide shorts YouTube Bromide Ion Silver silver bromide (agbr) is a chemical compound of silver and bromine. Learn more about its properties,. silver bromide is a pale yellow crystalline solid with the formula agbr. silver chloride dissolves readily in ammonia solution, while the bromide partially dissolves and the iodide does not. silver bromide is a pale yellow compound, which upon exposure to. Bromide Ion Silver.
From www.hatiandskoll.com
What is a silver halide? Hati and Skoll Gallery Bromide Ion Silver It has a rock salt structure and is light sensitive. learn how to identify halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. See the reactions, products, solubility products and. silver bromide is a pale yellow compound, which upon exposure to light, darkens due to the photochemical decomposition that. Learn how to prepare it, its.. Bromide Ion Silver.
From depositphotos.com
Silver bromide molecular structure — Stock Photo © sciencepics 75126675 Bromide Ion Silver It has a molecular weight of 187.772 and a cas. Adding iron(ii) solution to the silver. silver bromide is a pale yellow crystalline solid with the formula agbr. silver bromide (agbr) is a chemical compound of silver and bromine. Learn how to prepare it, its. Learn more about its properties,. It has a rock salt structure and is. Bromide Ion Silver.
From boomeria.org
Ch 25 Bromide Ion Silver Adding iron(ii) solution to the silver. It has a molecular weight of 187.772 and a cas. It has a rock salt structure and is light sensitive. silver bromide is a pale yellow compound, which upon exposure to light, darkens due to the photochemical decomposition that. Learn how to prepare it, its. silver bromide is a pale yellow crystalline. Bromide Ion Silver.
From sciencephoto.com
Silver bromide (AgBr) precipitate Stock Image A500/0642 Science Bromide Ion Silver Learn more about its properties,. silver chloride dissolves readily in ammonia solution, while the bromide partially dissolves and the iodide does not. Adding iron(ii) solution to the silver. silver bromide is a pale yellow crystalline solid with the formula agbr. It has a rock salt structure and is light sensitive. It has a molecular weight of 187.772 and. Bromide Ion Silver.
From www.nagwa.com
Question Video Writing a Net Ionic Equation for the Reaction between Bromide Ion Silver It has a rock salt structure and is light sensitive. silver chloride dissolves readily in ammonia solution, while the bromide partially dissolves and the iodide does not. learn how to identify halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. It has a molecular weight of 187.772 and a cas. Adding iron(ii) solution to the. Bromide Ion Silver.
From www.youtube.com
Silver bromide YouTube Bromide Ion Silver It has a molecular weight of 187.772 and a cas. learn how to identify halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. It has a rock salt structure and is light sensitive. silver chloride dissolves readily in ammonia solution, while the bromide partially dissolves and the iodide does not. silver bromide (agbr) is. Bromide Ion Silver.
From www.shutterstock.com
24 Silver Bromide Images, Stock Photos & Vectors Shutterstock Bromide Ion Silver silver bromide (agbr) is a chemical compound of silver and bromine. Learn more about its properties,. It has a molecular weight of 187.772 and a cas. silver bromide is a pale yellow compound, which upon exposure to light, darkens due to the photochemical decomposition that. It has a rock salt structure and is light sensitive. Adding iron(ii) solution. Bromide Ion Silver.
From www.toppr.com
Solubility product of silver bromide is 5.0 × 10^13 . The quantity of Bromide Ion Silver It has a molecular weight of 187.772 and a cas. learn how to identify halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. Adding iron(ii) solution to the silver. silver bromide (agbr) is a chemical compound of silver and bromine. silver bromide is a pale yellow crystalline solid with the formula agbr. See the. Bromide Ion Silver.
From fphoto.photoshelter.com
science chemistry precipitation reaction silver bromide Fundamental Bromide Ion Silver Learn how to prepare it, its. It has a molecular weight of 187.772 and a cas. silver chloride dissolves readily in ammonia solution, while the bromide partially dissolves and the iodide does not. It has a rock salt structure and is light sensitive. learn how to identify halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and. Bromide Ion Silver.
From www.researchgate.net
(PDF) Reaction of Bromous Acid with Bromide in the Presence of Silver Ions Bromide Ion Silver silver chloride dissolves readily in ammonia solution, while the bromide partially dissolves and the iodide does not. Learn more about its properties,. silver bromide is a pale yellow crystalline solid with the formula agbr. It has a rock salt structure and is light sensitive. Learn how to prepare it, its. silver bromide (agbr) is a chemical compound. Bromide Ion Silver.
From www.youtube.com
Quick video Balancing an oxidation reduction reaction in base [bromine Bromide Ion Silver See the reactions, products, solubility products and. silver bromide is a pale yellow crystalline solid with the formula agbr. silver chloride dissolves readily in ammonia solution, while the bromide partially dissolves and the iodide does not. It has a molecular weight of 187.772 and a cas. silver bromide is a pale yellow compound, which upon exposure to. Bromide Ion Silver.
From www.youtube.com
Equation for AgBr + H2O (Silver bromide + Water) YouTube Bromide Ion Silver silver chloride dissolves readily in ammonia solution, while the bromide partially dissolves and the iodide does not. Learn how to prepare it, its. It has a molecular weight of 187.772 and a cas. See the reactions, products, solubility products and. silver bromide (agbr) is a chemical compound of silver and bromine. silver bromide is a pale yellow. Bromide Ion Silver.
From exowfdyuj.blob.core.windows.net
Bromate Bromide Formation at Daisy Haven blog Bromide Ion Silver silver chloride dissolves readily in ammonia solution, while the bromide partially dissolves and the iodide does not. Learn more about its properties,. learn how to identify halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. Learn how to prepare it, its. Adding iron(ii) solution to the silver. silver bromide (agbr) is a chemical compound. Bromide Ion Silver.
From www.numerade.com
SOLVED write balanced equation for the following reaction first Silver Bromide Ion Silver silver bromide is a pale yellow crystalline solid with the formula agbr. Learn how to prepare it, its. silver bromide is a pale yellow compound, which upon exposure to light, darkens due to the photochemical decomposition that. silver chloride dissolves readily in ammonia solution, while the bromide partially dissolves and the iodide does not. Learn more about. Bromide Ion Silver.
From edu.svet.gob.gt
Bromide Ions And Silver Nitrate edu.svet.gob.gt Bromide Ion Silver silver bromide is a pale yellow crystalline solid with the formula agbr. silver chloride dissolves readily in ammonia solution, while the bromide partially dissolves and the iodide does not. silver bromide is a pale yellow compound, which upon exposure to light, darkens due to the photochemical decomposition that. silver bromide (agbr) is a chemical compound of. Bromide Ion Silver.
From www.fishersci.ie
Silver bromide, 99.9, Alfa Aesar™ Other Compounds Chemicals Bromide Ion Silver It has a rock salt structure and is light sensitive. silver bromide is a pale yellow crystalline solid with the formula agbr. silver chloride dissolves readily in ammonia solution, while the bromide partially dissolves and the iodide does not. See the reactions, products, solubility products and. It has a molecular weight of 187.772 and a cas. Adding iron(ii). Bromide Ion Silver.
From www.youtube.com
Sodium Bromide and Silver Nitrate YouTube Bromide Ion Silver It has a molecular weight of 187.772 and a cas. See the reactions, products, solubility products and. silver bromide is a pale yellow crystalline solid with the formula agbr. learn how to identify halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. silver chloride dissolves readily in ammonia solution, while the bromide partially dissolves. Bromide Ion Silver.
From dxodjrzxh.blob.core.windows.net
Bromide Ion Experiment at Frank Radcliff blog Bromide Ion Silver silver bromide is a pale yellow compound, which upon exposure to light, darkens due to the photochemical decomposition that. It has a molecular weight of 187.772 and a cas. Learn how to prepare it, its. It has a rock salt structure and is light sensitive. Learn more about its properties,. silver chloride dissolves readily in ammonia solution, while. Bromide Ion Silver.
From edu.svet.gob.gt
Bromide Ions And Silver Nitrate edu.svet.gob.gt Bromide Ion Silver silver bromide is a pale yellow crystalline solid with the formula agbr. It has a molecular weight of 187.772 and a cas. silver bromide is a pale yellow compound, which upon exposure to light, darkens due to the photochemical decomposition that. Adding iron(ii) solution to the silver. learn how to identify halide ions (fluoride, chloride, bromide and. Bromide Ion Silver.
From pixels.com
Silver Bromide Precipitation Photograph by Martyn F. Chillmaid/science Bromide Ion Silver See the reactions, products, solubility products and. silver bromide is a pale yellow compound, which upon exposure to light, darkens due to the photochemical decomposition that. silver bromide (agbr) is a chemical compound of silver and bromine. Learn how to prepare it, its. learn how to identify halide ions (fluoride, chloride, bromide and iodide) using silver nitrate. Bromide Ion Silver.
From www.youtube.com
How to Draw the Lewis Dot Structure for Br (Bromide ion) YouTube Bromide Ion Silver See the reactions, products, solubility products and. It has a rock salt structure and is light sensitive. It has a molecular weight of 187.772 and a cas. silver bromide (agbr) is a chemical compound of silver and bromine. silver chloride dissolves readily in ammonia solution, while the bromide partially dissolves and the iodide does not. silver bromide. Bromide Ion Silver.
From www.teachoo.com
What type of reaction takes place when silver bromide is exposed to Bromide Ion Silver Adding iron(ii) solution to the silver. silver bromide (agbr) is a chemical compound of silver and bromine. learn how to identify halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. silver bromide is a pale yellow compound, which upon exposure to light, darkens due to the photochemical decomposition that. See the reactions, products, solubility. Bromide Ion Silver.
From pubs.acs.org
Silver Bromide Nanoparticle/Polymer Composites Dual Action Tunable Bromide Ion Silver Learn how to prepare it, its. silver bromide (agbr) is a chemical compound of silver and bromine. See the reactions, products, solubility products and. silver bromide is a pale yellow compound, which upon exposure to light, darkens due to the photochemical decomposition that. silver chloride dissolves readily in ammonia solution, while the bromide partially dissolves and the. Bromide Ion Silver.
From www.sciencephoto.com
Test tube of silver bromide. Stock Image C029/1110 Science Photo Bromide Ion Silver silver chloride dissolves readily in ammonia solution, while the bromide partially dissolves and the iodide does not. Adding iron(ii) solution to the silver. Learn how to prepare it, its. learn how to identify halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. silver bromide is a pale yellow crystalline solid with the formula agbr.. Bromide Ion Silver.
From ask.modifiyegaraj.com
What Best Describes The Bromide Ion That Forms Asking List Bromide Ion Silver Learn more about its properties,. learn how to identify halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. silver bromide is a pale yellow compound, which upon exposure to light, darkens due to the photochemical decomposition that. silver bromide (agbr) is a chemical compound of silver and bromine. See the reactions, products, solubility products. Bromide Ion Silver.
From www.film-photography-blog.com
Film processing chemistry, how does it work? Film Photography Blog Bromide Ion Silver silver bromide is a pale yellow crystalline solid with the formula agbr. It has a molecular weight of 187.772 and a cas. It has a rock salt structure and is light sensitive. See the reactions, products, solubility products and. Adding iron(ii) solution to the silver. silver chloride dissolves readily in ammonia solution, while the bromide partially dissolves and. Bromide Ion Silver.
From www.youtube.com
Balance AgNO3 + NaBr = NaNO3 + AgBr (Silver Nitrate and Sodium Bromide Bromide Ion Silver It has a rock salt structure and is light sensitive. silver bromide is a pale yellow crystalline solid with the formula agbr. learn how to identify halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. Adding iron(ii) solution to the silver. silver bromide is a pale yellow compound, which upon exposure to light, darkens. Bromide Ion Silver.
From www.numerade.com
SOLVED Consider the reaction of silver ion with bromide ion given Bromide Ion Silver Adding iron(ii) solution to the silver. Learn how to prepare it, its. silver bromide is a pale yellow crystalline solid with the formula agbr. See the reactions, products, solubility products and. silver chloride dissolves readily in ammonia solution, while the bromide partially dissolves and the iodide does not. It has a molecular weight of 187.772 and a cas.. Bromide Ion Silver.
From www.alamy.com
Silver compounds precipitated in test tubes. From left to right, these Bromide Ion Silver See the reactions, products, solubility products and. silver bromide is a pale yellow compound, which upon exposure to light, darkens due to the photochemical decomposition that. silver bromide is a pale yellow crystalline solid with the formula agbr. Learn more about its properties,. It has a molecular weight of 187.772 and a cas. It has a rock salt. Bromide Ion Silver.
From www.nanochemazone.com
Silver Bromide Powder Low Price 1 Highly pure Nanochemazone Bromide Ion Silver silver bromide (agbr) is a chemical compound of silver and bromine. Adding iron(ii) solution to the silver. silver bromide is a pale yellow compound, which upon exposure to light, darkens due to the photochemical decomposition that. silver bromide is a pale yellow crystalline solid with the formula agbr. It has a molecular weight of 187.772 and a. Bromide Ion Silver.