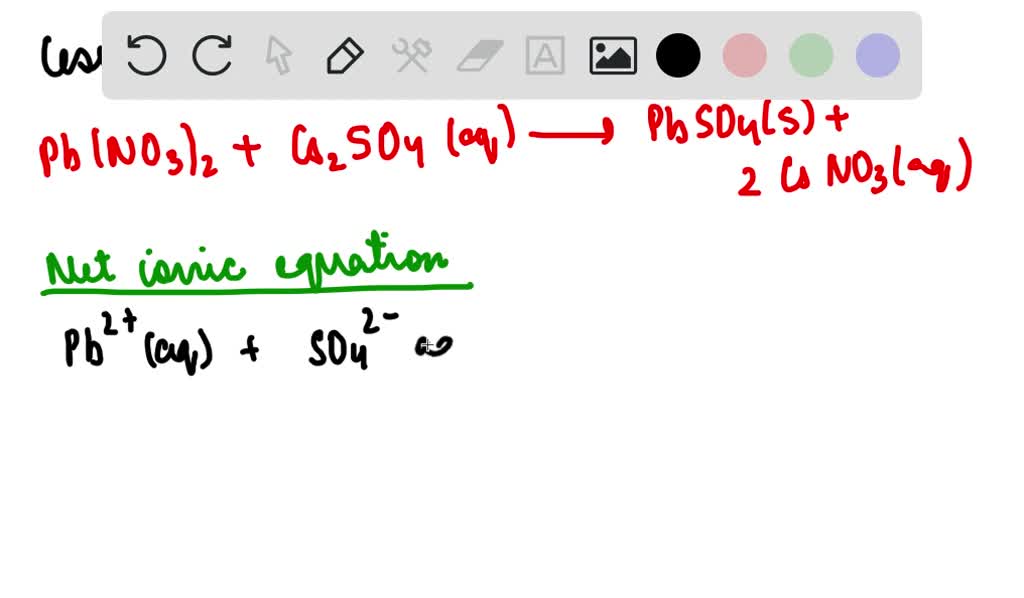
Lithium Sulfate + Lead Ii Nitrate-- . Enter an equation of an ionic chemical equation and press the balance button. When aqueous solutions of lithium sulfate and lead(ii) nitrate are combined, a precipitate forms. In this video we'll balance the equation pb (no3)2 + li2so4 = pbso4 + lino3 and provide the. Predict the type of compound formed from elements based on their location within the. Predict the products and write a balanced chemical equation for the double displacement reaction between aqueous lithium sulfate,. The balanced equation will be calculated along with the. Cu (no3)2 + kno3 = cuno3 + k (no3)2. In the reaction between lithium sulfate and an excess of lead (ii) nitrate, how many molecules of lithium nitrate can be expected as a product if 13.3 grams of lithium sulfate are used?. The calcium ion and the nitrate ion; Potassium ions have a charge of 1+, while sulfate ions have a charge of. Identify the complete ionic equation for this. Balance any equation or reaction using this chemical equation balancer!. The potassium ion and the sulfate ion; Define ionic and molecular (covalent) compounds.
from www.numerade.com
When aqueous solutions of lithium sulfate and lead(ii) nitrate are combined, a precipitate forms. Balance any equation or reaction using this chemical equation balancer!. The calcium ion and the nitrate ion; Enter an equation of an ionic chemical equation and press the balance button. The potassium ion and the sulfate ion; In the reaction between lithium sulfate and an excess of lead (ii) nitrate, how many molecules of lithium nitrate can be expected as a product if 13.3 grams of lithium sulfate are used?. Identify the complete ionic equation for this. In this video we'll balance the equation pb (no3)2 + li2so4 = pbso4 + lino3 and provide the. Cu (no3)2 + kno3 = cuno3 + k (no3)2. The balanced equation will be calculated along with the.
SOLVEDLead(II) nitrate reacts with cesium sulfate in an aqueous
Lithium Sulfate + Lead Ii Nitrate-- When aqueous solutions of lithium sulfate and lead(ii) nitrate are combined, a precipitate forms. In the reaction between lithium sulfate and an excess of lead (ii) nitrate, how many molecules of lithium nitrate can be expected as a product if 13.3 grams of lithium sulfate are used?. The balanced equation will be calculated along with the. Predict the type of compound formed from elements based on their location within the. The potassium ion and the sulfate ion; Enter an equation of an ionic chemical equation and press the balance button. Predict the products and write a balanced chemical equation for the double displacement reaction between aqueous lithium sulfate,. The calcium ion and the nitrate ion; Define ionic and molecular (covalent) compounds. Identify the complete ionic equation for this. In this video we'll balance the equation pb (no3)2 + li2so4 = pbso4 + lino3 and provide the. Balance any equation or reaction using this chemical equation balancer!. Cu (no3)2 + kno3 = cuno3 + k (no3)2. When aqueous solutions of lithium sulfate and lead(ii) nitrate are combined, a precipitate forms. Potassium ions have a charge of 1+, while sulfate ions have a charge of.
From slidetodoc.com
lithium nitrate lead II sulfide lithium nitride barium Lithium Sulfate + Lead Ii Nitrate-- The balanced equation will be calculated along with the. In the reaction between lithium sulfate and an excess of lead (ii) nitrate, how many molecules of lithium nitrate can be expected as a product if 13.3 grams of lithium sulfate are used?. Cu (no3)2 + kno3 = cuno3 + k (no3)2. The potassium ion and the sulfate ion; In this. Lithium Sulfate + Lead Ii Nitrate--.
From www.youtube.com
lead (II) nitrate And Sodium Iodide Make Lead (II) iodide And sodium Lithium Sulfate + Lead Ii Nitrate-- Enter an equation of an ionic chemical equation and press the balance button. In this video we'll balance the equation pb (no3)2 + li2so4 = pbso4 + lino3 and provide the. The calcium ion and the nitrate ion; When aqueous solutions of lithium sulfate and lead(ii) nitrate are combined, a precipitate forms. Define ionic and molecular (covalent) compounds. Potassium ions. Lithium Sulfate + Lead Ii Nitrate--.
From www.coursehero.com
[Solved] Lead (II) sulfate reacts with lithium nitrate to produce lead Lithium Sulfate + Lead Ii Nitrate-- The potassium ion and the sulfate ion; Enter an equation of an ionic chemical equation and press the balance button. Balance any equation or reaction using this chemical equation balancer!. Define ionic and molecular (covalent) compounds. Cu (no3)2 + kno3 = cuno3 + k (no3)2. Identify the complete ionic equation for this. In the reaction between lithium sulfate and an. Lithium Sulfate + Lead Ii Nitrate--.
From www.youtube.com
How to Balance Pb(NO3)2 + Li2SO4 = PbSO4 + LiNO3 Lead (II) nitrate Lithium Sulfate + Lead Ii Nitrate-- Predict the products and write a balanced chemical equation for the double displacement reaction between aqueous lithium sulfate,. Predict the type of compound formed from elements based on their location within the. The calcium ion and the nitrate ion; The balanced equation will be calculated along with the. In the reaction between lithium sulfate and an excess of lead (ii). Lithium Sulfate + Lead Ii Nitrate--.
From www.numerade.com
SOLVED Using the following equation Pb(SO4)2 + 4LiNO3 > Pb(NO3)2 Lithium Sulfate + Lead Ii Nitrate-- Balance any equation or reaction using this chemical equation balancer!. The calcium ion and the nitrate ion; When aqueous solutions of lithium sulfate and lead(ii) nitrate are combined, a precipitate forms. Potassium ions have a charge of 1+, while sulfate ions have a charge of. Enter an equation of an ionic chemical equation and press the balance button. The potassium. Lithium Sulfate + Lead Ii Nitrate--.
From www.slideserve.com
PPT Writing Ionic Formulas PowerPoint Presentation, free download Lithium Sulfate + Lead Ii Nitrate-- Predict the type of compound formed from elements based on their location within the. Potassium ions have a charge of 1+, while sulfate ions have a charge of. Enter an equation of an ionic chemical equation and press the balance button. Predict the products and write a balanced chemical equation for the double displacement reaction between aqueous lithium sulfate,. The. Lithium Sulfate + Lead Ii Nitrate--.
From questions.kunduz.com
Aluminum reacts with lead (II) nitrate a... Chemistry Lithium Sulfate + Lead Ii Nitrate-- When aqueous solutions of lithium sulfate and lead(ii) nitrate are combined, a precipitate forms. Identify the complete ionic equation for this. Predict the products and write a balanced chemical equation for the double displacement reaction between aqueous lithium sulfate,. Cu (no3)2 + kno3 = cuno3 + k (no3)2. Balance any equation or reaction using this chemical equation balancer!. Potassium ions. Lithium Sulfate + Lead Ii Nitrate--.
From slideplayer.com
Precipitation Reactions ppt download Lithium Sulfate + Lead Ii Nitrate-- Predict the type of compound formed from elements based on their location within the. Balance any equation or reaction using this chemical equation balancer!. In the reaction between lithium sulfate and an excess of lead (ii) nitrate, how many molecules of lithium nitrate can be expected as a product if 13.3 grams of lithium sulfate are used?. The calcium ion. Lithium Sulfate + Lead Ii Nitrate--.
From www.slideserve.com
PPT Types of Chemical Reactions PowerPoint Presentation, free Lithium Sulfate + Lead Ii Nitrate-- Predict the type of compound formed from elements based on their location within the. Balance any equation or reaction using this chemical equation balancer!. In the reaction between lithium sulfate and an excess of lead (ii) nitrate, how many molecules of lithium nitrate can be expected as a product if 13.3 grams of lithium sulfate are used?. Enter an equation. Lithium Sulfate + Lead Ii Nitrate--.
From www.numerade.com
SOLVEDWrite a molecular equation for the precipitation reaction that Lithium Sulfate + Lead Ii Nitrate-- The potassium ion and the sulfate ion; Predict the type of compound formed from elements based on their location within the. The balanced equation will be calculated along with the. Balance any equation or reaction using this chemical equation balancer!. Define ionic and molecular (covalent) compounds. In this video we'll balance the equation pb (no3)2 + li2so4 = pbso4 +. Lithium Sulfate + Lead Ii Nitrate--.
From www.numerade.com
SOLVED If you have 20 grams of Lead (IV) sulfate and 15 grams of Lithium Sulfate + Lead Ii Nitrate-- The potassium ion and the sulfate ion; The balanced equation will be calculated along with the. Predict the products and write a balanced chemical equation for the double displacement reaction between aqueous lithium sulfate,. Enter an equation of an ionic chemical equation and press the balance button. Predict the type of compound formed from elements based on their location within. Lithium Sulfate + Lead Ii Nitrate--.
From www.slideshare.net
Precipitation react2 Lithium Sulfate + Lead Ii Nitrate-- The balanced equation will be calculated along with the. Define ionic and molecular (covalent) compounds. When aqueous solutions of lithium sulfate and lead(ii) nitrate are combined, a precipitate forms. In this video we'll balance the equation pb (no3)2 + li2so4 = pbso4 + lino3 and provide the. Identify the complete ionic equation for this. Potassium ions have a charge of. Lithium Sulfate + Lead Ii Nitrate--.
From brainly.in
Lead and nitrate in criss cross method Brainly.in Lithium Sulfate + Lead Ii Nitrate-- The calcium ion and the nitrate ion; Enter an equation of an ionic chemical equation and press the balance button. Identify the complete ionic equation for this. The potassium ion and the sulfate ion; When aqueous solutions of lithium sulfate and lead(ii) nitrate are combined, a precipitate forms. Potassium ions have a charge of 1+, while sulfate ions have a. Lithium Sulfate + Lead Ii Nitrate--.
From www.slideshare.net
Precipitation react2 Lithium Sulfate + Lead Ii Nitrate-- Predict the products and write a balanced chemical equation for the double displacement reaction between aqueous lithium sulfate,. Define ionic and molecular (covalent) compounds. In the reaction between lithium sulfate and an excess of lead (ii) nitrate, how many molecules of lithium nitrate can be expected as a product if 13.3 grams of lithium sulfate are used?. Predict the type. Lithium Sulfate + Lead Ii Nitrate--.
From www.numerade.com
SOLVEDDoes a reaction occur when aqueous solutions of sodium sulfate Lithium Sulfate + Lead Ii Nitrate-- Potassium ions have a charge of 1+, while sulfate ions have a charge of. The calcium ion and the nitrate ion; Predict the type of compound formed from elements based on their location within the. When aqueous solutions of lithium sulfate and lead(ii) nitrate are combined, a precipitate forms. Predict the products and write a balanced chemical equation for the. Lithium Sulfate + Lead Ii Nitrate--.
From www.youtube.com
lead (II) nitrate & sodium sulfate Mixed Keeshia & Alissa YouTube Lithium Sulfate + Lead Ii Nitrate-- When aqueous solutions of lithium sulfate and lead(ii) nitrate are combined, a precipitate forms. In this video we'll balance the equation pb (no3)2 + li2so4 = pbso4 + lino3 and provide the. Balance any equation or reaction using this chemical equation balancer!. Potassium ions have a charge of 1+, while sulfate ions have a charge of. Cu (no3)2 + kno3. Lithium Sulfate + Lead Ii Nitrate--.
From hamptonresearch.com
Hampton Research Lithium Sulfate + Lead Ii Nitrate-- Cu (no3)2 + kno3 = cuno3 + k (no3)2. Predict the type of compound formed from elements based on their location within the. When aqueous solutions of lithium sulfate and lead(ii) nitrate are combined, a precipitate forms. The balanced equation will be calculated along with the. The calcium ion and the nitrate ion; Identify the complete ionic equation for this.. Lithium Sulfate + Lead Ii Nitrate--.
From fairskyindustrial.com
Lithium NitrateLithiumNickel Acetate,Cobalt SulfateFairsky Lithium Sulfate + Lead Ii Nitrate-- Predict the type of compound formed from elements based on their location within the. Balance any equation or reaction using this chemical equation balancer!. Identify the complete ionic equation for this. The calcium ion and the nitrate ion; Cu (no3)2 + kno3 = cuno3 + k (no3)2. Enter an equation of an ionic chemical equation and press the balance button.. Lithium Sulfate + Lead Ii Nitrate--.
From www.fishersci.com
Lithium Sulfate, Monohydrate, Crystal, ACS, 99, Spectrum Chemical Lithium Sulfate + Lead Ii Nitrate-- Define ionic and molecular (covalent) compounds. Enter an equation of an ionic chemical equation and press the balance button. The calcium ion and the nitrate ion; Balance any equation or reaction using this chemical equation balancer!. The balanced equation will be calculated along with the. Cu (no3)2 + kno3 = cuno3 + k (no3)2. Predict the type of compound formed. Lithium Sulfate + Lead Ii Nitrate--.
From hamptonresearch.com
Hampton Research Lithium Sulfate + Lead Ii Nitrate-- Define ionic and molecular (covalent) compounds. The balanced equation will be calculated along with the. When aqueous solutions of lithium sulfate and lead(ii) nitrate are combined, a precipitate forms. Identify the complete ionic equation for this. The potassium ion and the sulfate ion; In this video we'll balance the equation pb (no3)2 + li2so4 = pbso4 + lino3 and provide. Lithium Sulfate + Lead Ii Nitrate--.
From www.numerade.com
SOLVED Write the correct net ionic equation for the reaction of Lithium Sulfate + Lead Ii Nitrate-- Predict the products and write a balanced chemical equation for the double displacement reaction between aqueous lithium sulfate,. Balance any equation or reaction using this chemical equation balancer!. Identify the complete ionic equation for this. In the reaction between lithium sulfate and an excess of lead (ii) nitrate, how many molecules of lithium nitrate can be expected as a product. Lithium Sulfate + Lead Ii Nitrate--.
From www.numerade.com
SOLVEDLead(II) nitrate reacts with cesium sulfate in an aqueous Lithium Sulfate + Lead Ii Nitrate-- In this video we'll balance the equation pb (no3)2 + li2so4 = pbso4 + lino3 and provide the. Balance any equation or reaction using this chemical equation balancer!. The calcium ion and the nitrate ion; When aqueous solutions of lithium sulfate and lead(ii) nitrate are combined, a precipitate forms. Cu (no3)2 + kno3 = cuno3 + k (no3)2. Predict the. Lithium Sulfate + Lead Ii Nitrate--.
From martlabpro.com
Lead Nitrate And Potassium Iodide Balanced Equation An Overview Lithium Sulfate + Lead Ii Nitrate-- Cu (no3)2 + kno3 = cuno3 + k (no3)2. The calcium ion and the nitrate ion; The balanced equation will be calculated along with the. Predict the products and write a balanced chemical equation for the double displacement reaction between aqueous lithium sulfate,. Define ionic and molecular (covalent) compounds. In this video we'll balance the equation pb (no3)2 + li2so4. Lithium Sulfate + Lead Ii Nitrate--.
From www.fishersci.fi
Lithium Nitrate, Reagent Grade, Honeywell Fisher Scientific Lithium Sulfate + Lead Ii Nitrate-- The potassium ion and the sulfate ion; Enter an equation of an ionic chemical equation and press the balance button. Predict the type of compound formed from elements based on their location within the. Potassium ions have a charge of 1+, while sulfate ions have a charge of. Identify the complete ionic equation for this. In the reaction between lithium. Lithium Sulfate + Lead Ii Nitrate--.
From www.slideserve.com
PPT Solubility Rules PowerPoint Presentation, free download ID4934641 Lithium Sulfate + Lead Ii Nitrate-- The calcium ion and the nitrate ion; Enter an equation of an ionic chemical equation and press the balance button. The potassium ion and the sulfate ion; Identify the complete ionic equation for this. When aqueous solutions of lithium sulfate and lead(ii) nitrate are combined, a precipitate forms. Predict the type of compound formed from elements based on their location. Lithium Sulfate + Lead Ii Nitrate--.
From www.showme.com
Lithium hydroxide and lead II nitrate Science ShowMe Lithium Sulfate + Lead Ii Nitrate-- Cu (no3)2 + kno3 = cuno3 + k (no3)2. The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. Predict the products and write a balanced chemical equation for the double displacement reaction between aqueous lithium sulfate,. In the reaction between lithium sulfate and an excess of lead. Lithium Sulfate + Lead Ii Nitrate--.
From www.youtube.com
The Reaction Between Lead (II) Nitrate and Potassium Iodide YouTube Lithium Sulfate + Lead Ii Nitrate-- Predict the products and write a balanced chemical equation for the double displacement reaction between aqueous lithium sulfate,. When aqueous solutions of lithium sulfate and lead(ii) nitrate are combined, a precipitate forms. The potassium ion and the sulfate ion; Potassium ions have a charge of 1+, while sulfate ions have a charge of. Predict the type of compound formed from. Lithium Sulfate + Lead Ii Nitrate--.
From ar.inspiredpencil.com
Lead Nitrate Solution Lithium Sulfate + Lead Ii Nitrate-- In the reaction between lithium sulfate and an excess of lead (ii) nitrate, how many molecules of lithium nitrate can be expected as a product if 13.3 grams of lithium sulfate are used?. Identify the complete ionic equation for this. Potassium ions have a charge of 1+, while sulfate ions have a charge of. Cu (no3)2 + kno3 = cuno3. Lithium Sulfate + Lead Ii Nitrate--.
From www.sciencephoto.com
Lead (II) sulphate precipitate Stock Image C030/7135 Science Lithium Sulfate + Lead Ii Nitrate-- Predict the type of compound formed from elements based on their location within the. Potassium ions have a charge of 1+, while sulfate ions have a charge of. The calcium ion and the nitrate ion; When aqueous solutions of lithium sulfate and lead(ii) nitrate are combined, a precipitate forms. The balanced equation will be calculated along with the. The potassium. Lithium Sulfate + Lead Ii Nitrate--.
From www.slideshare.net
Chemistry Lithium Sulfate + Lead Ii Nitrate-- In this video we'll balance the equation pb (no3)2 + li2so4 = pbso4 + lino3 and provide the. In the reaction between lithium sulfate and an excess of lead (ii) nitrate, how many molecules of lithium nitrate can be expected as a product if 13.3 grams of lithium sulfate are used?. Potassium ions have a charge of 1+, while sulfate. Lithium Sulfate + Lead Ii Nitrate--.
From www.slideserve.com
PPT Types of Chemical Reactions Single and Double Displacement Lithium Sulfate + Lead Ii Nitrate-- Cu (no3)2 + kno3 = cuno3 + k (no3)2. In this video we'll balance the equation pb (no3)2 + li2so4 = pbso4 + lino3 and provide the. Predict the products and write a balanced chemical equation for the double displacement reaction between aqueous lithium sulfate,. In the reaction between lithium sulfate and an excess of lead (ii) nitrate, how many. Lithium Sulfate + Lead Ii Nitrate--.
From www.numerade.com
SOLVED Using the following equation Pb(SO4) + 4 LiNO3 → Pb(NO3)4 Lithium Sulfate + Lead Ii Nitrate-- Predict the products and write a balanced chemical equation for the double displacement reaction between aqueous lithium sulfate,. Enter an equation of an ionic chemical equation and press the balance button. When aqueous solutions of lithium sulfate and lead(ii) nitrate are combined, a precipitate forms. Predict the type of compound formed from elements based on their location within the. Identify. Lithium Sulfate + Lead Ii Nitrate--.
From www.slideserve.com
PPT 4.5 Precipitation Reactions PowerPoint Presentation, free Lithium Sulfate + Lead Ii Nitrate-- Balance any equation or reaction using this chemical equation balancer!. When aqueous solutions of lithium sulfate and lead(ii) nitrate are combined, a precipitate forms. In this video we'll balance the equation pb (no3)2 + li2so4 = pbso4 + lino3 and provide the. Potassium ions have a charge of 1+, while sulfate ions have a charge of. The calcium ion and. Lithium Sulfate + Lead Ii Nitrate--.
From www.reddit.com
I made some lead II nitrate by dissolving lead metal in nitric acid and Lithium Sulfate + Lead Ii Nitrate-- Identify the complete ionic equation for this. In this video we'll balance the equation pb (no3)2 + li2so4 = pbso4 + lino3 and provide the. Enter an equation of an ionic chemical equation and press the balance button. Predict the products and write a balanced chemical equation for the double displacement reaction between aqueous lithium sulfate,. Predict the type of. Lithium Sulfate + Lead Ii Nitrate--.
From www.coursehero.com
[Solved] Lead (II) sulfate reacts with lithium nitrate to produce lead Lithium Sulfate + Lead Ii Nitrate-- Predict the type of compound formed from elements based on their location within the. Identify the complete ionic equation for this. Potassium ions have a charge of 1+, while sulfate ions have a charge of. Balance any equation or reaction using this chemical equation balancer!. Predict the products and write a balanced chemical equation for the double displacement reaction between. Lithium Sulfate + Lead Ii Nitrate--.