Standard Heat Of Formation Practice Problems . The standard heat of combustion of benzene is −3271 kj/mol, for co 2 it is −394 kj/mol, and for h 2 o, it is −286 kj/mol. Assume both the reactants and products are under standard state conditions, and that the heat released is directly proportional to the enthalpy. This online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of chemical. Practice calculating the standard heat of formation with practice problems and explanations. In this set of practice questions, we will summarize the main concepts of thermochemistry such as the relationship between internal energy, work and heat, exothermic and endothermic. Get instant feedback, extra help. The heat of combustion of propane. What is the balanced equation for the combustion reaction of diisopropyl ether (c 6 h 14 o), a gasoline additive that has a standard enthalpy. Write a balanced equation for the complete combustion of propane gas, which yields co2(g) and h2o(l).
from askfilo.com
In this set of practice questions, we will summarize the main concepts of thermochemistry such as the relationship between internal energy, work and heat, exothermic and endothermic. The standard heat of combustion of benzene is −3271 kj/mol, for co 2 it is −394 kj/mol, and for h 2 o, it is −286 kj/mol. Assume both the reactants and products are under standard state conditions, and that the heat released is directly proportional to the enthalpy. This online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of chemical. Get instant feedback, extra help. Write a balanced equation for the complete combustion of propane gas, which yields co2(g) and h2o(l). The heat of combustion of propane. Practice calculating the standard heat of formation with practice problems and explanations. What is the balanced equation for the combustion reaction of diisopropyl ether (c 6 h 14 o), a gasoline additive that has a standard enthalpy.
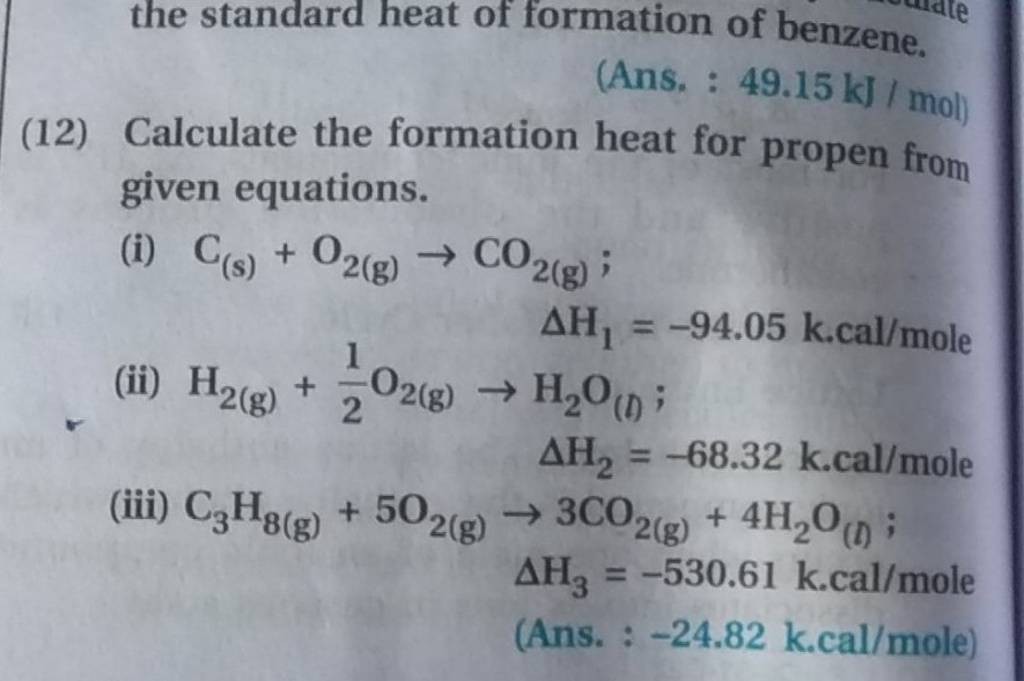
the standard heat of formation of benzene. (Ans. 49.15 kJ/mol ) (12) Ca..
Standard Heat Of Formation Practice Problems Write a balanced equation for the complete combustion of propane gas, which yields co2(g) and h2o(l). This online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of chemical. Get instant feedback, extra help. In this set of practice questions, we will summarize the main concepts of thermochemistry such as the relationship between internal energy, work and heat, exothermic and endothermic. Practice calculating the standard heat of formation with practice problems and explanations. The standard heat of combustion of benzene is −3271 kj/mol, for co 2 it is −394 kj/mol, and for h 2 o, it is −286 kj/mol. Write a balanced equation for the complete combustion of propane gas, which yields co2(g) and h2o(l). The heat of combustion of propane. What is the balanced equation for the combustion reaction of diisopropyl ether (c 6 h 14 o), a gasoline additive that has a standard enthalpy. Assume both the reactants and products are under standard state conditions, and that the heat released is directly proportional to the enthalpy.
From www.youtube.com
Standard Enthalpies of Formation Problem (Chemistry) YouTube Standard Heat Of Formation Practice Problems Assume both the reactants and products are under standard state conditions, and that the heat released is directly proportional to the enthalpy. The heat of combustion of propane. This online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of chemical. What is the balanced equation for the combustion reaction. Standard Heat Of Formation Practice Problems.
From byjus.com
43. Calculate standard heat of formation of CS2. Given that standard Standard Heat Of Formation Practice Problems Assume both the reactants and products are under standard state conditions, and that the heat released is directly proportional to the enthalpy. The heat of combustion of propane. This online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of chemical. Practice calculating the standard heat of formation with practice. Standard Heat Of Formation Practice Problems.
From www.chegg.com
Solved The standard heat of formation, ΔH∘f, is defined as Standard Heat Of Formation Practice Problems This online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of chemical. The heat of combustion of propane. What is the balanced equation for the combustion reaction of diisopropyl ether (c 6 h 14 o), a gasoline additive that has a standard enthalpy. Practice calculating the standard heat of. Standard Heat Of Formation Practice Problems.
From www.toppr.com
The standard heat of formation of NO2(g) and N2O4(g) are 8.0 and 4.0 Standard Heat Of Formation Practice Problems This online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of chemical. The heat of combustion of propane. In this set of practice questions, we will summarize the main concepts of thermochemistry such as the relationship between internal energy, work and heat, exothermic and endothermic. Practice calculating the standard. Standard Heat Of Formation Practice Problems.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Standard Heat Of Formation Practice Problems Write a balanced equation for the complete combustion of propane gas, which yields co2(g) and h2o(l). What is the balanced equation for the combustion reaction of diisopropyl ether (c 6 h 14 o), a gasoline additive that has a standard enthalpy. Assume both the reactants and products are under standard state conditions, and that the heat released is directly proportional. Standard Heat Of Formation Practice Problems.
From www.youtube.com
Standard Heat of Formation YouTube Standard Heat Of Formation Practice Problems This online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of chemical. Assume both the reactants and products are under standard state conditions, and that the heat released is directly proportional to the enthalpy. In this set of practice questions, we will summarize the main concepts of thermochemistry such. Standard Heat Of Formation Practice Problems.
From askfilo.com
the standard heat of formation of benzene. (Ans. 49.15 kJ/mol ) (12) Ca.. Standard Heat Of Formation Practice Problems This online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of chemical. In this set of practice questions, we will summarize the main concepts of thermochemistry such as the relationship between internal energy, work and heat, exothermic and endothermic. Practice calculating the standard heat of formation with practice problems. Standard Heat Of Formation Practice Problems.
From www.chegg.com
Solved The standard heat of formation, ΔHf, is defined as Standard Heat Of Formation Practice Problems In this set of practice questions, we will summarize the main concepts of thermochemistry such as the relationship between internal energy, work and heat, exothermic and endothermic. Practice calculating the standard heat of formation with practice problems and explanations. Write a balanced equation for the complete combustion of propane gas, which yields co2(g) and h2o(l). What is the balanced equation. Standard Heat Of Formation Practice Problems.
From www.slideserve.com
PPT Chemistry 17.4 PowerPoint Presentation, free download ID2772524 Standard Heat Of Formation Practice Problems This online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of chemical. In this set of practice questions, we will summarize the main concepts of thermochemistry such as the relationship between internal energy, work and heat, exothermic and endothermic. What is the balanced equation for the combustion reaction of. Standard Heat Of Formation Practice Problems.
From www.chegg.com
Solved The standard heat of formation for BaO(s) is −554 Standard Heat Of Formation Practice Problems In this set of practice questions, we will summarize the main concepts of thermochemistry such as the relationship between internal energy, work and heat, exothermic and endothermic. This online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of chemical. Assume both the reactants and products are under standard state. Standard Heat Of Formation Practice Problems.
From www.chegg.com
Solved The standard heat of formation for BaO(s) is −554 Standard Heat Of Formation Practice Problems Practice calculating the standard heat of formation with practice problems and explanations. Assume both the reactants and products are under standard state conditions, and that the heat released is directly proportional to the enthalpy. Get instant feedback, extra help. The heat of combustion of propane. This online quiz is intended to give you extra practice in hess's law problems using. Standard Heat Of Formation Practice Problems.
From www.studocu.com
Heats of formation worksheet key Name Standard Heats of Formation Standard Heat Of Formation Practice Problems Assume both the reactants and products are under standard state conditions, and that the heat released is directly proportional to the enthalpy. Practice calculating the standard heat of formation with practice problems and explanations. This online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of chemical. What is the. Standard Heat Of Formation Practice Problems.
From www.chegg.com
Solved D Hess's Law Calculations Use the standard heats of Standard Heat Of Formation Practice Problems Assume both the reactants and products are under standard state conditions, and that the heat released is directly proportional to the enthalpy. In this set of practice questions, we will summarize the main concepts of thermochemistry such as the relationship between internal energy, work and heat, exothermic and endothermic. The heat of combustion of propane. The standard heat of combustion. Standard Heat Of Formation Practice Problems.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF Standard Heat Of Formation Practice Problems The standard heat of combustion of benzene is −3271 kj/mol, for co 2 it is −394 kj/mol, and for h 2 o, it is −286 kj/mol. The heat of combustion of propane. Practice calculating the standard heat of formation with practice problems and explanations. This online quiz is intended to give you extra practice in hess's law problems using standard. Standard Heat Of Formation Practice Problems.
From www.chegg.com
Solved Problem 2 The standard heat of formation for some Standard Heat Of Formation Practice Problems Write a balanced equation for the complete combustion of propane gas, which yields co2(g) and h2o(l). Practice calculating the standard heat of formation with practice problems and explanations. Get instant feedback, extra help. This online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of chemical. What is the balanced. Standard Heat Of Formation Practice Problems.
From www.chegg.com
Solved What is the standard heat of formation for ammonium Standard Heat Of Formation Practice Problems The standard heat of combustion of benzene is −3271 kj/mol, for co 2 it is −394 kj/mol, and for h 2 o, it is −286 kj/mol. Assume both the reactants and products are under standard state conditions, and that the heat released is directly proportional to the enthalpy. Write a balanced equation for the complete combustion of propane gas, which. Standard Heat Of Formation Practice Problems.
From www.chegg.com
Solved 13. The standard enthalpies of formation for several Standard Heat Of Formation Practice Problems What is the balanced equation for the combustion reaction of diisopropyl ether (c 6 h 14 o), a gasoline additive that has a standard enthalpy. Assume both the reactants and products are under standard state conditions, and that the heat released is directly proportional to the enthalpy. The standard heat of combustion of benzene is −3271 kj/mol, for co 2. Standard Heat Of Formation Practice Problems.
From askfilo.com
Standard heat of formation of CH4 ( g), CO2 ( g) and water at 25∘C are −1.. Standard Heat Of Formation Practice Problems The heat of combustion of propane. What is the balanced equation for the combustion reaction of diisopropyl ether (c 6 h 14 o), a gasoline additive that has a standard enthalpy. In this set of practice questions, we will summarize the main concepts of thermochemistry such as the relationship between internal energy, work and heat, exothermic and endothermic. The standard. Standard Heat Of Formation Practice Problems.
From www.youtube.com
How to Calculate Enthalpy of Reaction using Heat of Formation Examples Standard Heat Of Formation Practice Problems Write a balanced equation for the complete combustion of propane gas, which yields co2(g) and h2o(l). Practice calculating the standard heat of formation with practice problems and explanations. The standard heat of combustion of benzene is −3271 kj/mol, for co 2 it is −394 kj/mol, and for h 2 o, it is −286 kj/mol. What is the balanced equation for. Standard Heat Of Formation Practice Problems.
From rayb78.github.io
Heat Of Formation Chart Standard Heat Of Formation Practice Problems Write a balanced equation for the complete combustion of propane gas, which yields co2(g) and h2o(l). The standard heat of combustion of benzene is −3271 kj/mol, for co 2 it is −394 kj/mol, and for h 2 o, it is −286 kj/mol. The heat of combustion of propane. Assume both the reactants and products are under standard state conditions, and. Standard Heat Of Formation Practice Problems.
From www.nagwa.com
Question Video Calculating the Standard Enthalpy of Formation for Standard Heat Of Formation Practice Problems Assume both the reactants and products are under standard state conditions, and that the heat released is directly proportional to the enthalpy. In this set of practice questions, we will summarize the main concepts of thermochemistry such as the relationship between internal energy, work and heat, exothermic and endothermic. Practice calculating the standard heat of formation with practice problems and. Standard Heat Of Formation Practice Problems.
From www.studocu.com
Calorimetry Calorimetry Formation Reactions The standard heat of Standard Heat Of Formation Practice Problems Practice calculating the standard heat of formation with practice problems and explanations. This online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of chemical. In this set of practice questions, we will summarize the main concepts of thermochemistry such as the relationship between internal energy, work and heat, exothermic. Standard Heat Of Formation Practice Problems.
From www.youtube.com
5.1 Standard enthalpy changes of formation and combustion YouTube Standard Heat Of Formation Practice Problems In this set of practice questions, we will summarize the main concepts of thermochemistry such as the relationship between internal energy, work and heat, exothermic and endothermic. Write a balanced equation for the complete combustion of propane gas, which yields co2(g) and h2o(l). What is the balanced equation for the combustion reaction of diisopropyl ether (c 6 h 14 o),. Standard Heat Of Formation Practice Problems.
From www.slideserve.com
PPT Standard Heats of Reaction PowerPoint Presentation, free download Standard Heat Of Formation Practice Problems Practice calculating the standard heat of formation with practice problems and explanations. This online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of chemical. The heat of combustion of propane. Write a balanced equation for the complete combustion of propane gas, which yields co2(g) and h2o(l). Get instant feedback,. Standard Heat Of Formation Practice Problems.
From www.coursehero.com
[Solved] 1. All of the following compounds have a standard heat of Standard Heat Of Formation Practice Problems The standard heat of combustion of benzene is −3271 kj/mol, for co 2 it is −394 kj/mol, and for h 2 o, it is −286 kj/mol. What is the balanced equation for the combustion reaction of diisopropyl ether (c 6 h 14 o), a gasoline additive that has a standard enthalpy. The heat of combustion of propane. Write a balanced. Standard Heat Of Formation Practice Problems.
From www.youtube.com
🔺️H for the reaction, F2 + 2HCl 》2HF +Cl2 is 352.8kj and standard Standard Heat Of Formation Practice Problems The heat of combustion of propane. Assume both the reactants and products are under standard state conditions, and that the heat released is directly proportional to the enthalpy. The standard heat of combustion of benzene is −3271 kj/mol, for co 2 it is −394 kj/mol, and for h 2 o, it is −286 kj/mol. Write a balanced equation for the. Standard Heat Of Formation Practice Problems.
From ar.inspiredpencil.com
Heat Of Formation Table Standard Heat Of Formation Practice Problems The standard heat of combustion of benzene is −3271 kj/mol, for co 2 it is −394 kj/mol, and for h 2 o, it is −286 kj/mol. What is the balanced equation for the combustion reaction of diisopropyl ether (c 6 h 14 o), a gasoline additive that has a standard enthalpy. In this set of practice questions, we will summarize. Standard Heat Of Formation Practice Problems.
From www.youtube.com
Heat of Reaction (from Heat of Formation) YouTube Standard Heat Of Formation Practice Problems Assume both the reactants and products are under standard state conditions, and that the heat released is directly proportional to the enthalpy. The heat of combustion of propane. Get instant feedback, extra help. The standard heat of combustion of benzene is −3271 kj/mol, for co 2 it is −394 kj/mol, and for h 2 o, it is −286 kj/mol. Write. Standard Heat Of Formation Practice Problems.
From www.chegg.com
The standard heat of formation, ΔrH∘, is defined as Standard Heat Of Formation Practice Problems Practice calculating the standard heat of formation with practice problems and explanations. Assume both the reactants and products are under standard state conditions, and that the heat released is directly proportional to the enthalpy. The standard heat of combustion of benzene is −3271 kj/mol, for co 2 it is −394 kj/mol, and for h 2 o, it is −286 kj/mol.. Standard Heat Of Formation Practice Problems.
From www.chegg.com
Solved Use and interpret standard heats of formation. (a) Standard Heat Of Formation Practice Problems Assume both the reactants and products are under standard state conditions, and that the heat released is directly proportional to the enthalpy. In this set of practice questions, we will summarize the main concepts of thermochemistry such as the relationship between internal energy, work and heat, exothermic and endothermic. This online quiz is intended to give you extra practice in. Standard Heat Of Formation Practice Problems.
From www.chegg.com
Solved The standard heat of formation for CaCl2 (s) is −796 Standard Heat Of Formation Practice Problems Assume both the reactants and products are under standard state conditions, and that the heat released is directly proportional to the enthalpy. The heat of combustion of propane. What is the balanced equation for the combustion reaction of diisopropyl ether (c 6 h 14 o), a gasoline additive that has a standard enthalpy. This online quiz is intended to give. Standard Heat Of Formation Practice Problems.
From www.youtube.com
Enthalpy of Formation Reaction & Heat of Combustion, Enthalpy Change Standard Heat Of Formation Practice Problems Write a balanced equation for the complete combustion of propane gas, which yields co2(g) and h2o(l). In this set of practice questions, we will summarize the main concepts of thermochemistry such as the relationship between internal energy, work and heat, exothermic and endothermic. Assume both the reactants and products are under standard state conditions, and that the heat released is. Standard Heat Of Formation Practice Problems.
From general.chemistrysteps.com
Thermochemistry Practice Problems Chemistry Steps Standard Heat Of Formation Practice Problems The heat of combustion of propane. Practice calculating the standard heat of formation with practice problems and explanations. In this set of practice questions, we will summarize the main concepts of thermochemistry such as the relationship between internal energy, work and heat, exothermic and endothermic. Get instant feedback, extra help. What is the balanced equation for the combustion reaction of. Standard Heat Of Formation Practice Problems.
From www.coursehero.com
[Solved] 1. All of the following compounds have a standard heat of Standard Heat Of Formation Practice Problems Write a balanced equation for the complete combustion of propane gas, which yields co2(g) and h2o(l). Practice calculating the standard heat of formation with practice problems and explanations. The standard heat of combustion of benzene is −3271 kj/mol, for co 2 it is −394 kj/mol, and for h 2 o, it is −286 kj/mol. This online quiz is intended to. Standard Heat Of Formation Practice Problems.
From miracleabbcortez.blogspot.com
Heat of Reaction Formula MiracleabbCortez Standard Heat Of Formation Practice Problems Write a balanced equation for the complete combustion of propane gas, which yields co2(g) and h2o(l). The standard heat of combustion of benzene is −3271 kj/mol, for co 2 it is −394 kj/mol, and for h 2 o, it is −286 kj/mol. Assume both the reactants and products are under standard state conditions, and that the heat released is directly. Standard Heat Of Formation Practice Problems.