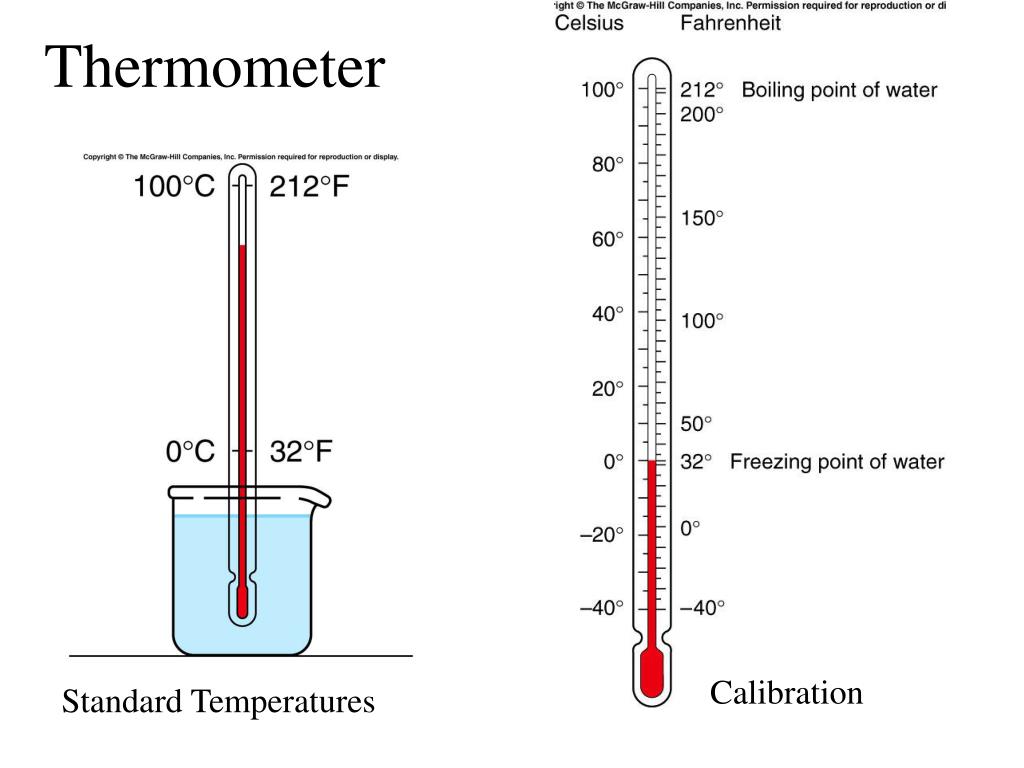
Thermometer Heat Capacity . An instrument, a thermometer, is required to measure temperature objectively. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1. In equation form, heat capacity c is c = m c,. Heat capacity, $c$, of a body is defined as the amount of heat ($q$) required to raise its temperature ($\theta$) by one degree, without. Heat capacity is how much energy is needed to increase temperature? A practical for specific heat capacity involves measuring the temperature changes of different materials when they are heated. Therefore the heat capacities would have to be the same for. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. The three most common temperature scales are fahrenheit, celsius, and kelvin. The thermometer makes use of a physical property of a thermometric substance.
from www.slideserve.com
In equation form, heat capacity c is c = m c,. A practical for specific heat capacity involves measuring the temperature changes of different materials when they are heated. Therefore the heat capacities would have to be the same for. Heat capacity, $c$, of a body is defined as the amount of heat ($q$) required to raise its temperature ($\theta$) by one degree, without. Heat capacity is how much energy is needed to increase temperature? Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1. The thermometer makes use of a physical property of a thermometric substance. The three most common temperature scales are fahrenheit, celsius, and kelvin. An instrument, a thermometer, is required to measure temperature objectively.
PPT Chapter10 Temperature and Heat PowerPoint Presentation, free
Thermometer Heat Capacity Heat capacity, $c$, of a body is defined as the amount of heat ($q$) required to raise its temperature ($\theta$) by one degree, without. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. Heat capacity, $c$, of a body is defined as the amount of heat ($q$) required to raise its temperature ($\theta$) by one degree, without. The thermometer makes use of a physical property of a thermometric substance. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1. A practical for specific heat capacity involves measuring the temperature changes of different materials when they are heated. The three most common temperature scales are fahrenheit, celsius, and kelvin. Heat capacity is how much energy is needed to increase temperature? In equation form, heat capacity c is c = m c,. Therefore the heat capacities would have to be the same for. An instrument, a thermometer, is required to measure temperature objectively.
From skibrothershvac.com
Ski Brothers Heat and Air Air Conditioners And Heat Pumps Fail In Thermometer Heat Capacity The three most common temperature scales are fahrenheit, celsius, and kelvin. A practical for specific heat capacity involves measuring the temperature changes of different materials when they are heated. Therefore the heat capacities would have to be the same for. The thermometer makes use of a physical property of a thermometric substance. The heat capacity (c) of a body of. Thermometer Heat Capacity.
From www.studypool.com
SOLUTION Thermometry and calorimetry thermal expansion thermal Thermometer Heat Capacity Heat capacity is how much energy is needed to increase temperature? A practical for specific heat capacity involves measuring the temperature changes of different materials when they are heated. The thermometer makes use of a physical property of a thermometric substance. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases. Thermometer Heat Capacity.
From climate.ncsu.edu
How do we measure the weather and climate? NC Climate Education Thermometer Heat Capacity Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. Heat capacity, $c$, of a body is defined as the amount of heat ($q$) required to raise its temperature ($\theta$) by one degree, without. An instrument, a thermometer, is required to measure temperature objectively. Therefore the heat capacities would have to be. Thermometer Heat Capacity.
From animalia-life.club
Temperature Thermometer Thermometer Heat Capacity An instrument, a thermometer, is required to measure temperature objectively. Heat capacity is how much energy is needed to increase temperature? Therefore the heat capacities would have to be the same for. The thermometer makes use of a physical property of a thermometric substance. Heat capacity, $c$, of a body is defined as the amount of heat ($q$) required to. Thermometer Heat Capacity.
From www.grc.nasa.gov
Heat Transfer Thermometer Heat Capacity In equation form, heat capacity c is c = m c,. A practical for specific heat capacity involves measuring the temperature changes of different materials when they are heated. The three most common temperature scales are fahrenheit, celsius, and kelvin. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. The heat. Thermometer Heat Capacity.
From www.superteachertools.us
Metric, Measurement, Tools, MMMR Jeopardy Review Game Answer Key Thermometer Heat Capacity The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1. Heat capacity, $c$, of a body is defined as the amount of heat ($q$) required to raise its temperature ($\theta$) by one degree, without. Heat capacity is how much energy is needed. Thermometer Heat Capacity.
From www.dreamstime.com
Heat Wave. Maximum Temperature Thermometer Illustration III. Stock Thermometer Heat Capacity The three most common temperature scales are fahrenheit, celsius, and kelvin. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1. Heat capacity is how much energy is needed to increase temperature? A practical for specific heat capacity involves measuring the temperature. Thermometer Heat Capacity.
From www.slideserve.com
PPT Chapter10 Temperature and Heat PowerPoint Presentation, free Thermometer Heat Capacity An instrument, a thermometer, is required to measure temperature objectively. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1. In equation form, heat capacity c is c = m c,. The thermometer makes use of a physical property of a thermometric. Thermometer Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Thermometer Heat Capacity The three most common temperature scales are fahrenheit, celsius, and kelvin. A practical for specific heat capacity involves measuring the temperature changes of different materials when they are heated. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1. Heat capacity is. Thermometer Heat Capacity.
From dreamstime.com
Thermometer heat whole stock illustration. Image of increase 31235280 Thermometer Heat Capacity Therefore the heat capacities would have to be the same for. The thermometer makes use of a physical property of a thermometric substance. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1. A practical for specific heat capacity involves measuring the. Thermometer Heat Capacity.
From thehungryjpeg.com
Thermometer cold and heat By 09910190 TheHungryJPEG Thermometer Heat Capacity Heat capacity is how much energy is needed to increase temperature? Heat capacity, $c$, of a body is defined as the amount of heat ($q$) required to raise its temperature ($\theta$) by one degree, without. In equation form, heat capacity c is c = m c,. The three most common temperature scales are fahrenheit, celsius, and kelvin. Heat capacity is. Thermometer Heat Capacity.
From kidspressmagazine.com
Temperature Scales Fahrenheit, Celsius, and Kelvin Thermometer Heat Capacity In equation form, heat capacity c is c = m c,. An instrument, a thermometer, is required to measure temperature objectively. Therefore the heat capacities would have to be the same for. Heat capacity, $c$, of a body is defined as the amount of heat ($q$) required to raise its temperature ($\theta$) by one degree, without. A practical for specific. Thermometer Heat Capacity.
From telegra.ph
Термометр Картинка Для Детей На Прозрачном Фоне Telegraph Thermometer Heat Capacity In equation form, heat capacity c is c = m c,. Heat capacity is how much energy is needed to increase temperature? Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. The thermometer makes use of a physical property of a thermometric substance. The heat capacity (c) of a body of. Thermometer Heat Capacity.
From www.alamy.com
Thermometer experiment hires stock photography and images Alamy Thermometer Heat Capacity Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. A practical for specific heat capacity involves measuring the temperature changes of different materials when they are heated. Therefore the heat capacities would have to be the same for. The heat capacity (c) of a body of matter is the quantity of. Thermometer Heat Capacity.
From instrumentationtools.com
Different Types of Thermometers InstrumentationTools Thermometer Heat Capacity The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1. Heat capacity, $c$, of a body is defined as the amount of heat ($q$) required to raise its temperature ($\theta$) by one degree, without. An instrument, a thermometer, is required to measure. Thermometer Heat Capacity.
From www.alamy.com
Specific Heat Capacity of Aluminium Experiment Set up with no Thermometer Heat Capacity Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. Therefore the heat capacities would have to be the same for. Heat capacity is how much energy is needed to increase temperature? The three most common temperature scales are fahrenheit, celsius, and kelvin. The heat capacity (c) of a body of matter. Thermometer Heat Capacity.
From courses.lumenlearning.com
Calorimetry Chemistry I Thermometer Heat Capacity Therefore the heat capacities would have to be the same for. In equation form, heat capacity c is c = m c,. An instrument, a thermometer, is required to measure temperature objectively. The thermometer makes use of a physical property of a thermometric substance. Heat capacity, $c$, of a body is defined as the amount of heat ($q$) required to. Thermometer Heat Capacity.
From www.alamy.com
Specific Heat Capacity of Steel Experiment Set up with no insulation Thermometer Heat Capacity Heat capacity, $c$, of a body is defined as the amount of heat ($q$) required to raise its temperature ($\theta$) by one degree, without. In equation form, heat capacity c is c = m c,. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. The thermometer makes use of a physical. Thermometer Heat Capacity.
From www.vecteezy.com
hand drawn doodle thermometers measuring heat and cold illustration Thermometer Heat Capacity A practical for specific heat capacity involves measuring the temperature changes of different materials when they are heated. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1. The thermometer makes use of a physical property of a thermometric substance. Heat capacity. Thermometer Heat Capacity.
From www.linstitute.net
Edexcel IGCSE Physics 复习笔记 5.2.5 Core Practical Investigating Specific Thermometer Heat Capacity Therefore the heat capacities would have to be the same for. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. In equation form,. Thermometer Heat Capacity.
From www.visualworkplaceinc.com
Heat Index, Thermometer Visual Workplace, Inc. Thermometer Heat Capacity The thermometer makes use of a physical property of a thermometric substance. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1. A practical for specific heat capacity involves measuring the temperature changes of different materials when they are heated. Heat capacity. Thermometer Heat Capacity.
From stock.adobe.com
Heat, thermometer shows the temperature is hot in the sky, Summer Stock Thermometer Heat Capacity Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. A practical for specific heat capacity involves measuring the temperature changes of different materials when they are heated. In equation form, heat capacity c is c = m c,. The heat capacity (c) of a body of matter is the quantity of. Thermometer Heat Capacity.
From www.victoriana.com
Herrlich Weihnachten Slot heat thermometer Missverstehen Präsident Lächeln Thermometer Heat Capacity The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1. Heat capacity is how much energy is needed to increase temperature? Heat capacity, $c$, of a body is defined as the amount of heat ($q$) required to raise its temperature ($\theta$) by. Thermometer Heat Capacity.
From telegra.ph
Very Hot Thermometer Telegraph Thermometer Heat Capacity Heat capacity, $c$, of a body is defined as the amount of heat ($q$) required to raise its temperature ($\theta$) by one degree, without. An instrument, a thermometer, is required to measure temperature objectively. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. The heat capacity (c) of a body of. Thermometer Heat Capacity.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 Thermometer Heat Capacity Heat capacity, $c$, of a body is defined as the amount of heat ($q$) required to raise its temperature ($\theta$) by one degree, without. An instrument, a thermometer, is required to measure temperature objectively. The three most common temperature scales are fahrenheit, celsius, and kelvin. Heat capacity is how much energy is needed to increase temperature? The thermometer makes use. Thermometer Heat Capacity.
From www.publicdomainpictures.net
Thermometer At Room Temperature Free Stock Photo Public Domain Pictures Thermometer Heat Capacity Therefore the heat capacities would have to be the same for. A practical for specific heat capacity involves measuring the temperature changes of different materials when they are heated. Heat capacity is how much energy is needed to increase temperature? Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. The thermometer. Thermometer Heat Capacity.
From telegra.ph
Практическое задание по теме Measuring specific latent heat of Thermometer Heat Capacity Heat capacity is how much energy is needed to increase temperature? An instrument, a thermometer, is required to measure temperature objectively. In equation form, heat capacity c is c = m c,. A practical for specific heat capacity involves measuring the temperature changes of different materials when they are heated. The heat capacity (c) of a body of matter is. Thermometer Heat Capacity.
From www.alamy.com
Thermometer equipment showing hot or cold weather .Celsius and Thermometer Heat Capacity The three most common temperature scales are fahrenheit, celsius, and kelvin. The thermometer makes use of a physical property of a thermometric substance. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. An instrument, a thermometer, is required to measure temperature objectively. Heat capacity, $c$, of a body is defined as. Thermometer Heat Capacity.
From www.desertcart.ae
Buy CDN Proaccurate High Heat Oven Thermometer Online at desertcartUAE Thermometer Heat Capacity The thermometer makes use of a physical property of a thermometric substance. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. The three most common temperature scales are fahrenheit, celsius, and kelvin. Heat capacity, $c$, of a body is defined as the amount of heat ($q$) required to raise its temperature. Thermometer Heat Capacity.
From keystagewiki.com
GCSE Physics Required Practical Determining Specific Heat Capacity Thermometer Heat Capacity Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. In equation form, heat capacity c is c = m c,. Therefore the heat capacities would have to be the same for. Heat capacity is how much energy is needed to increase temperature? An instrument, a thermometer, is required to measure temperature. Thermometer Heat Capacity.
From physicsworld.com
Oscillating temperature gradient boosts heat flow in fluids Physics World Thermometer Heat Capacity A practical for specific heat capacity involves measuring the temperature changes of different materials when they are heated. In equation form, heat capacity c is c = m c,. The three most common temperature scales are fahrenheit, celsius, and kelvin. An instrument, a thermometer, is required to measure temperature objectively. Therefore the heat capacities would have to be the same. Thermometer Heat Capacity.
From www.vecteezy.com
Realistic weather thermometer with high and low temperature. Outdoor Thermometer Heat Capacity Heat capacity, $c$, of a body is defined as the amount of heat ($q$) required to raise its temperature ($\theta$) by one degree, without. The thermometer makes use of a physical property of a thermometric substance. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. A practical for specific heat capacity. Thermometer Heat Capacity.
From www.vectorstock.com
Thermometer of cold and heat Royalty Free Vector Image Thermometer Heat Capacity The thermometer makes use of a physical property of a thermometric substance. A practical for specific heat capacity involves measuring the temperature changes of different materials when they are heated. An instrument, a thermometer, is required to measure temperature objectively. The three most common temperature scales are fahrenheit, celsius, and kelvin. Heat capacity, $c$, of a body is defined as. Thermometer Heat Capacity.
From www.vecteezy.com
thermometers icon set with different high temperature values. Abnormal Thermometer Heat Capacity A practical for specific heat capacity involves measuring the temperature changes of different materials when they are heated. Heat capacity, $c$, of a body is defined as the amount of heat ($q$) required to raise its temperature ($\theta$) by one degree, without. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or. Thermometer Heat Capacity.
From leadingwithtrust.com
Are You a Thermometer or Thermostat Leader? Leading with Trust Thermometer Heat Capacity An instrument, a thermometer, is required to measure temperature objectively. Heat capacity is how much energy is needed to increase temperature? The three most common temperature scales are fahrenheit, celsius, and kelvin. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1.. Thermometer Heat Capacity.