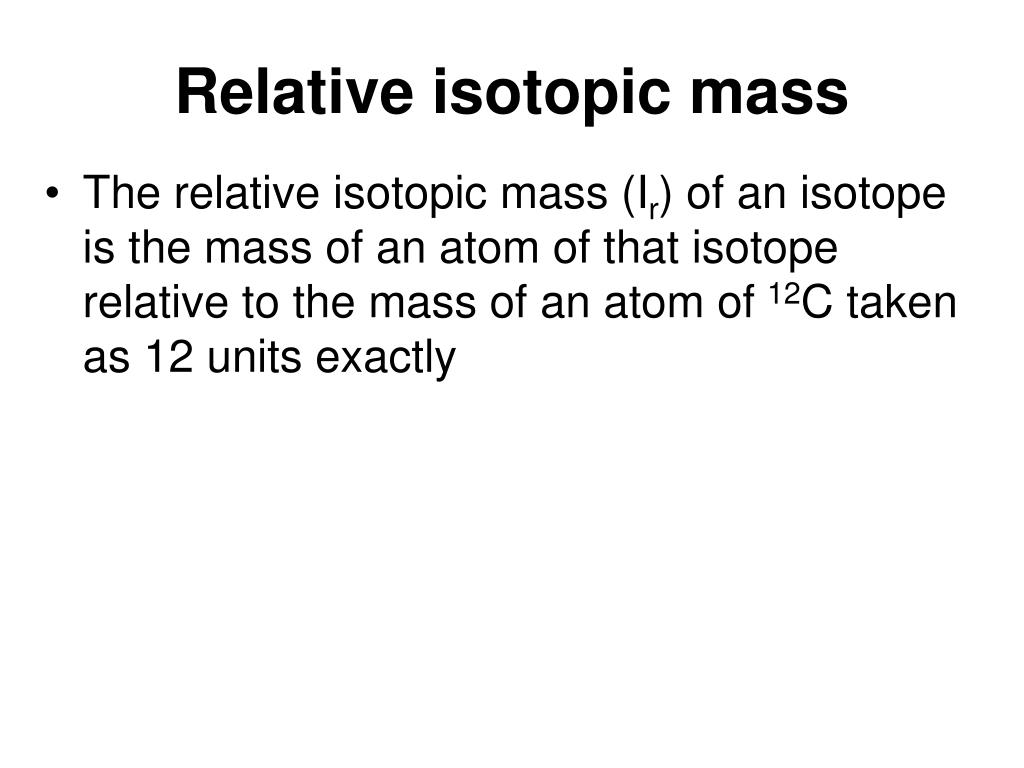
A Level Definition Of Relative Isotopic Mass . Because of this, the mass of an element is given as relative atomic. Relative molecular mass ( mr ) is defined as: The mean mass of a molecule of a compound, relative to one twelfth of the mean mass of an atom of. Atoms of the same element with a different number of neutrons. These are atoms of the same elements but with different mass numbers; The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. Relative atomic mass takes the relative abundances of the different isotopes of an element into account: The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit.
from www.slideserve.com
The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. Relative atomic mass takes the relative abundances of the different isotopes of an element into account: Atoms of the same element with a different number of neutrons. The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. Relative molecular mass ( mr ) is defined as: Because of this, the mass of an element is given as relative atomic. These are atoms of the same elements but with different mass numbers; The mean mass of a molecule of a compound, relative to one twelfth of the mean mass of an atom of.
PPT The mass of particles PowerPoint Presentation, free download ID5480969
A Level Definition Of Relative Isotopic Mass Relative atomic mass takes the relative abundances of the different isotopes of an element into account: The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. Relative molecular mass ( mr ) is defined as: Relative atomic mass takes the relative abundances of the different isotopes of an element into account: These are atoms of the same elements but with different mass numbers; Atoms of the same element with a different number of neutrons. The mean mass of a molecule of a compound, relative to one twelfth of the mean mass of an atom of. The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. Because of this, the mass of an element is given as relative atomic.
From www.youtube.com
Relative Isotopic Mass and Mass Spectrometry (Part 1) YouTube A Level Definition Of Relative Isotopic Mass Relative atomic mass takes the relative abundances of the different isotopes of an element into account: Relative molecular mass ( mr ) is defined as: The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. Atoms of the same element with a different number of neutrons.. A Level Definition Of Relative Isotopic Mass.
From www.slideserve.com
PPT Atoms, Ions and Isotopes PowerPoint Presentation ID5401475 A Level Definition Of Relative Isotopic Mass The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. Relative molecular mass ( mr ) is defined as: The mean mass. A Level Definition Of Relative Isotopic Mass.
From www.slideserve.com
PPT The mass of particles PowerPoint Presentation, free download ID5480969 A Level Definition Of Relative Isotopic Mass Atoms of the same element with a different number of neutrons. The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. These are atoms of the same elements but with different mass numbers; The mean mass of a molecule of a compound, relative to one twelfth. A Level Definition Of Relative Isotopic Mass.
From www.youtube.com
Pre lesson 11 Relative isotopic mass YouTube A Level Definition Of Relative Isotopic Mass Because of this, the mass of an element is given as relative atomic. The mean mass of a molecule of a compound, relative to one twelfth of the mean mass of an atom of. The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. These are. A Level Definition Of Relative Isotopic Mass.
From mychemway.com
The relative of atomic mass and molecular mass — MyChemWay A Level Definition Of Relative Isotopic Mass Because of this, the mass of an element is given as relative atomic. The mean mass of a molecule of a compound, relative to one twelfth of the mean mass of an atom of. Relative molecular mass ( mr ) is defined as: These are atoms of the same elements but with different mass numbers; Atoms of the same element. A Level Definition Of Relative Isotopic Mass.
From www.youtube.com
Relative Isotopic Mass Revision for Chemistry ALevel and GCSE YouTube A Level Definition Of Relative Isotopic Mass These are atoms of the same elements but with different mass numbers; The mean mass of a molecule of a compound, relative to one twelfth of the mean mass of an atom of. Relative molecular mass ( mr ) is defined as: The relative isotopic mass is the mass of a particular atom of an isotope compared to the value. A Level Definition Of Relative Isotopic Mass.
From www.tes.com
Isotopes and Relative Atomic Mass GCSE Lesson (SC3c CC3c) Teaching Resources A Level Definition Of Relative Isotopic Mass These are atoms of the same elements but with different mass numbers; The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. The mean mass of a molecule of a compound, relative to one twelfth of the mean mass of an atom of. Relative molecular mass. A Level Definition Of Relative Isotopic Mass.
From www.youtube.com
Calculating relative atomic mass YouTube A Level Definition Of Relative Isotopic Mass The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. Relative molecular mass ( mr ) is defined as: These are atoms of the same elements but with different mass numbers; The relative isotopic mass is the mass of a particular atom of an isotope compared. A Level Definition Of Relative Isotopic Mass.
From www.youtube.com
What is Relative Atomic Mass? Chemical Formula and Equation YouTube A Level Definition Of Relative Isotopic Mass The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. Relative molecular mass ( mr ) is defined as: Because of this, the mass of an element is given as relative atomic. Relative atomic mass takes the relative abundances of the different isotopes of an element. A Level Definition Of Relative Isotopic Mass.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo A Level Definition Of Relative Isotopic Mass Relative molecular mass ( mr ) is defined as: The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. Because of this, the mass of an element is given as relative atomic. These are atoms of the same elements but with different mass numbers; The mean. A Level Definition Of Relative Isotopic Mass.
From periodictable.me
How to Calculate Atomic Mass of Isotopes Archives Dynamic Periodic Table of Elements and Chemistry A Level Definition Of Relative Isotopic Mass Relative atomic mass takes the relative abundances of the different isotopes of an element into account: Relative molecular mass ( mr ) is defined as: Because of this, the mass of an element is given as relative atomic. Atoms of the same element with a different number of neutrons. The relative isotopic mass is the mass of a particular atom. A Level Definition Of Relative Isotopic Mass.
From animalia-life.club
Relative Molecular Mass A Level Definition Of Relative Isotopic Mass The mean mass of a molecule of a compound, relative to one twelfth of the mean mass of an atom of. Because of this, the mass of an element is given as relative atomic. The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. These are. A Level Definition Of Relative Isotopic Mass.
From www.youtube.com
A Level Chemistry Revision "Relative Atomic Mass" YouTube A Level Definition Of Relative Isotopic Mass The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. Relative atomic mass takes the relative abundances of the different isotopes of an element into account: The mean mass of a molecule of a compound, relative to one twelfth of the mean mass of an atom. A Level Definition Of Relative Isotopic Mass.
From atelier-yuwa.ciao.jp
Defining How To Calculate Relative Atomic Mass Of Element Relative Isotopic Mass Definition Gcse A Level Definition Of Relative Isotopic Mass The mean mass of a molecule of a compound, relative to one twelfth of the mean mass of an atom of. Relative molecular mass ( mr ) is defined as: Atoms of the same element with a different number of neutrons. The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of. A Level Definition Of Relative Isotopic Mass.
From www.slideserve.com
PPT The mass of particles PowerPoint Presentation, free download ID5480969 A Level Definition Of Relative Isotopic Mass These are atoms of the same elements but with different mass numbers; The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit.. A Level Definition Of Relative Isotopic Mass.
From www.slideserve.com
PPT Interpretation of Mass Spectra Part 4 PowerPoint Presentation, free download ID731720 A Level Definition Of Relative Isotopic Mass Relative atomic mass takes the relative abundances of the different isotopes of an element into account: The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the. A Level Definition Of Relative Isotopic Mass.
From maimelatct.com
ISOTOPE AND RELATIVE ATOMIC MASS Physical sciences break 1.0 A Level Definition Of Relative Isotopic Mass Because of this, the mass of an element is given as relative atomic. These are atoms of the same elements but with different mass numbers; Relative atomic mass takes the relative abundances of the different isotopes of an element into account: The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of. A Level Definition Of Relative Isotopic Mass.
From periodictable.me
Way to Find Atomic Mass of Elements Dynamic Periodic Table of Elements and Chemistry A Level Definition Of Relative Isotopic Mass These are atoms of the same elements but with different mass numbers; The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. Because of this, the mass of an element is given as relative atomic. Atoms of the same element with a different number of neutrons.. A Level Definition Of Relative Isotopic Mass.
From periodictable.me
How to Calculate Atomic Mass of Isotopes Archives Dynamic Periodic Table of Elements and Chemistry A Level Definition Of Relative Isotopic Mass The mean mass of a molecule of a compound, relative to one twelfth of the mean mass of an atom of. Relative atomic mass takes the relative abundances of the different isotopes of an element into account: Atoms of the same element with a different number of neutrons. Relative molecular mass ( mr ) is defined as: The relative isotopic. A Level Definition Of Relative Isotopic Mass.
From mavink.com
Relative Atomic Mass Formula Isotopes A Level Definition Of Relative Isotopic Mass The mean mass of a molecule of a compound, relative to one twelfth of the mean mass of an atom of. The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. Relative atomic mass takes the relative abundances of the different isotopes of an element into. A Level Definition Of Relative Isotopic Mass.
From atelier-yuwa.ciao.jp
Defining How To Calculate Relative Atomic Mass Of Element Relative Isotopic Mass Definition Gcse A Level Definition Of Relative Isotopic Mass The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. Relative molecular mass ( mr ) is defined as: The mean mass of a molecule of a compound, relative to one twelfth of the mean mass of an atom of. Because of this, the mass of. A Level Definition Of Relative Isotopic Mass.
From www.slideserve.com
PPT Finding An Isotopic Mass PowerPoint Presentation, free download ID6383465 A Level Definition Of Relative Isotopic Mass Relative molecular mass ( mr ) is defined as: The mean mass of a molecule of a compound, relative to one twelfth of the mean mass of an atom of. Relative atomic mass takes the relative abundances of the different isotopes of an element into account: The relative isotopic mass is the mass of a particular atom of an isotope. A Level Definition Of Relative Isotopic Mass.
From www.tes.com
Relative Atomic Mass OCR A Level Teaching Resources A Level Definition Of Relative Isotopic Mass Relative atomic mass takes the relative abundances of the different isotopes of an element into account: Atoms of the same element with a different number of neutrons. The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. Because of this, the mass of an element is. A Level Definition Of Relative Isotopic Mass.
From aschemistryrevisionforocr.blogspot.com
OCR AS Chemistry Revision Unit 1 Module 1 Atoms and Reactions A Level Definition Of Relative Isotopic Mass Because of this, the mass of an element is given as relative atomic. The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass. A Level Definition Of Relative Isotopic Mass.
From www.savemyexams.com
Isotopic Abundance & Relative Atomic Mass CIE A Level Chemistry Revision Notes 2022 A Level Definition Of Relative Isotopic Mass Atoms of the same element with a different number of neutrons. The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. Relative molecular mass ( mr ) is defined as: These are atoms of the same elements but with different mass numbers; The relative isotopic mass. A Level Definition Of Relative Isotopic Mass.
From www.youtube.com
1.2.1 Define the terms relative atomic mass (A r) and relative molecular mass (M r). YouTube A Level Definition Of Relative Isotopic Mass The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. The mean mass of a molecule of a compound, relative to one twelfth of the mean mass of an atom of. The relative isotopic mass is the mass of a particular atom of an isotope compared. A Level Definition Of Relative Isotopic Mass.
From www.slideserve.com
PPT Atomic Structure PowerPoint Presentation, free download ID4749012 A Level Definition Of Relative Isotopic Mass Relative molecular mass ( mr ) is defined as: Atoms of the same element with a different number of neutrons. The mean mass of a molecule of a compound, relative to one twelfth of the mean mass of an atom of. The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of. A Level Definition Of Relative Isotopic Mass.
From slidetodoc.com
3 Isotopes and Mass calculations Objectives Defining and A Level Definition Of Relative Isotopic Mass Atoms of the same element with a different number of neutrons. Relative atomic mass takes the relative abundances of the different isotopes of an element into account: The mean mass of a molecule of a compound, relative to one twelfth of the mean mass of an atom of. The relative isotopic mass is the mass of a particular atom of. A Level Definition Of Relative Isotopic Mass.
From www.youtube.com
Relative Isotopic Mass YouTube A Level Definition Of Relative Isotopic Mass Relative atomic mass takes the relative abundances of the different isotopes of an element into account: These are atoms of the same elements but with different mass numbers; Relative molecular mass ( mr ) is defined as: The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass. A Level Definition Of Relative Isotopic Mass.
From shiken.ai
Relative Atomic Mass A Level Definition Of Relative Isotopic Mass The mean mass of a molecule of a compound, relative to one twelfth of the mean mass of an atom of. Because of this, the mass of an element is given as relative atomic. The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. Relative molecular. A Level Definition Of Relative Isotopic Mass.
From www.youtube.com
SK015 CHAP 1 MATTER (PART 2 RELATIVE MASS & ISOTOPIC MASS) YouTube A Level Definition Of Relative Isotopic Mass Relative molecular mass ( mr ) is defined as: These are atoms of the same elements but with different mass numbers; Atoms of the same element with a different number of neutrons. The mean mass of a molecule of a compound, relative to one twelfth of the mean mass of an atom of. Relative atomic mass takes the relative abundances. A Level Definition Of Relative Isotopic Mass.
From www.youtube.com
Relative Molecular Mass & Relative Formula Mass YouTube A Level Definition Of Relative Isotopic Mass These are atoms of the same elements but with different mass numbers; Atoms of the same element with a different number of neutrons. The mean mass of a molecule of a compound, relative to one twelfth of the mean mass of an atom of. Relative atomic mass takes the relative abundances of the different isotopes of an element into account:. A Level Definition Of Relative Isotopic Mass.
From www.slideserve.com
PPT ATOMS, MOLECULES & STOICHIOMETRY PowerPoint Presentation ID4640698 A Level Definition Of Relative Isotopic Mass The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. Because of this, the mass of an element is given as relative atomic. Relative molecular mass ( mr ) is defined as: The mean mass of a molecule of a compound, relative to one twelfth of. A Level Definition Of Relative Isotopic Mass.
From www.youtube.com
Relative Atomic and Isotopic Mass Revision for ALevel Chemistry The Maths Bits YouTube A Level Definition Of Relative Isotopic Mass Because of this, the mass of an element is given as relative atomic. The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit. The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass. A Level Definition Of Relative Isotopic Mass.
From www.youtube.com
How to Calculate Relative Atomic Mass? Atomic v Isotopic Mass AS Level Conquer Chemistry A Level Definition Of Relative Isotopic Mass Atoms of the same element with a different number of neutrons. Because of this, the mass of an element is given as relative atomic. Relative molecular mass ( mr ) is defined as: Relative atomic mass takes the relative abundances of the different isotopes of an element into account: The mean mass of a molecule of a compound, relative to. A Level Definition Of Relative Isotopic Mass.