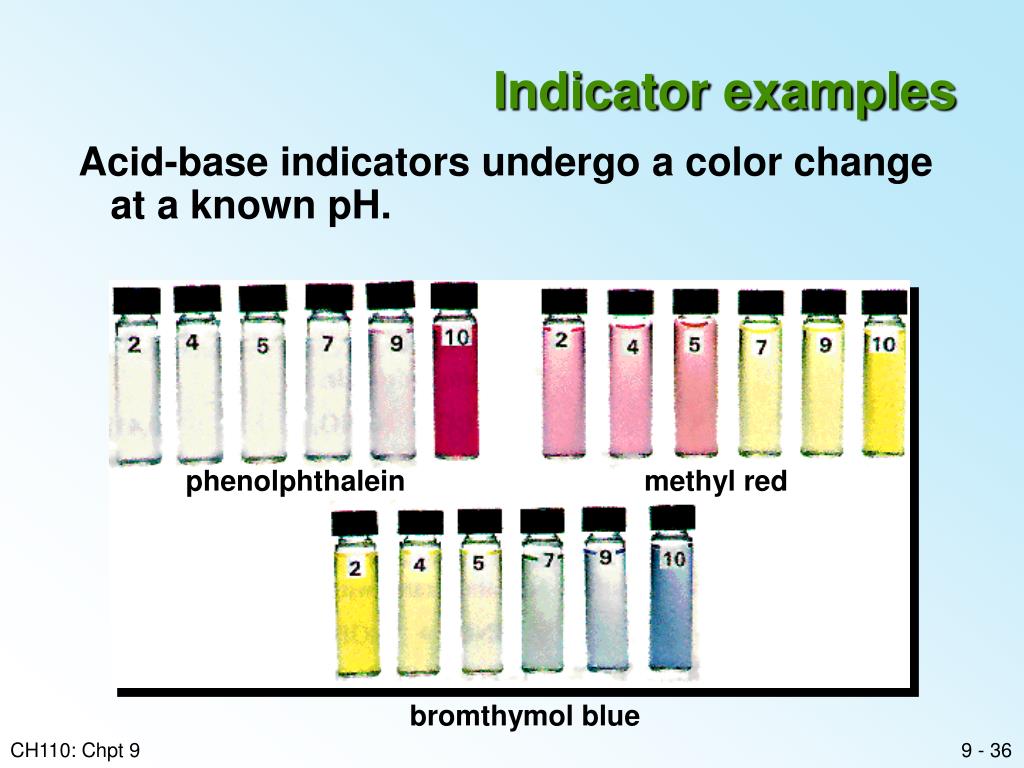
Basic Indicator Solution . A ph less than 7 indicates an acidic solution and a ph greater than 7 indicates a basic solution. Ultimately, the ph value indicates how much h +. Indicators are substances whose solutions change color due to changes in ph. The perceived color of an indicator solution is determined by the ratio of the concentrations of the two species in − and hin. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Only a small amount of indicator compound is. The quantity of indicator in. This page assumes that you know about ph curves for all the.
from www.slideserve.com
Only a small amount of indicator compound is. The perceived color of an indicator solution is determined by the ratio of the concentrations of the two species in − and hin. A ph less than 7 indicates an acidic solution and a ph greater than 7 indicates a basic solution. The quantity of indicator in. This page assumes that you know about ph curves for all the. Ultimately, the ph value indicates how much h +. Indicators are substances whose solutions change color due to changes in ph. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink.
PPT Acids and Bases PowerPoint Presentation, free download ID5741622
Basic Indicator Solution In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. A ph less than 7 indicates an acidic solution and a ph greater than 7 indicates a basic solution. Only a small amount of indicator compound is. Indicators are substances whose solutions change color due to changes in ph. The quantity of indicator in. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. This page assumes that you know about ph curves for all the. Ultimately, the ph value indicates how much h +. The perceived color of an indicator solution is determined by the ratio of the concentrations of the two species in − and hin.
From www.thoughtco.com
Definition and Examples of AcidBase Indicator Basic Indicator Solution The quantity of indicator in. A ph less than 7 indicates an acidic solution and a ph greater than 7 indicates a basic solution. Only a small amount of indicator compound is. Indicators are substances whose solutions change color due to changes in ph. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9. Basic Indicator Solution.
From sciencenotes.org
Universal Indicator Chart and Recipes Basic Indicator Solution The perceived color of an indicator solution is determined by the ratio of the concentrations of the two species in − and hin. The quantity of indicator in. This page assumes that you know about ph curves for all the. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3),. Basic Indicator Solution.
From www.youtube.com
Acid Base Test With China Rose Indicator Solution How To Make China Rose/Hibiscus Indicator Basic Indicator Solution In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. A ph less than 7 indicates an acidic solution and a ph greater than 7 indicates a basic solution. Ultimately, the ph value indicates how much h +. The quantity of indicator in. The perceived. Basic Indicator Solution.
From fphoto.photoshelter.com
science chemistry acid base indicator methyl red Fundamental Photographs The Art of Science Basic Indicator Solution Ultimately, the ph value indicates how much h +. A ph less than 7 indicates an acidic solution and a ph greater than 7 indicates a basic solution. The quantity of indicator in. This page assumes that you know about ph curves for all the. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10. Basic Indicator Solution.
From courses.lumenlearning.com
AcidBase Indicators Introduction to Chemistry Basic Indicator Solution Only a small amount of indicator compound is. Ultimately, the ph value indicates how much h +. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. A ph less than 7 indicates an acidic solution and a ph greater than 7 indicates a basic. Basic Indicator Solution.
From avopix.com
PH indicator. Acidic, neutral, basic solutions. Royalty Free Stock Vector 2263636707 Basic Indicator Solution Only a small amount of indicator compound is. The perceived color of an indicator solution is determined by the ratio of the concentrations of the two species in − and hin. Indicators are substances whose solutions change color due to changes in ph. The quantity of indicator in. A ph less than 7 indicates an acidic solution and a ph. Basic Indicator Solution.
From brunofuga.adv.br
Chemical Indicators Definition, Types And Examples, 56 OFF Basic Indicator Solution The quantity of indicator in. The perceived color of an indicator solution is determined by the ratio of the concentrations of the two species in − and hin. Indicators are substances whose solutions change color due to changes in ph. This page assumes that you know about ph curves for all the. Only a small amount of indicator compound is.. Basic Indicator Solution.
From dxokymive.blob.core.windows.net
Types Of Indicators In Acid Base Titration at Donna Gutierrez blog Basic Indicator Solution The quantity of indicator in. The perceived color of an indicator solution is determined by the ratio of the concentrations of the two species in − and hin. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. A ph less than 7 indicates an. Basic Indicator Solution.
From www.sciencephoto.com
Universal indicator solution and paper Stock Image C004/7681 Science Photo Library Basic Indicator Solution This page assumes that you know about ph curves for all the. Ultimately, the ph value indicates how much h +. Only a small amount of indicator compound is. Indicators are substances whose solutions change color due to changes in ph. A ph less than 7 indicates an acidic solution and a ph greater than 7 indicates a basic solution.. Basic Indicator Solution.
From stock.adobe.com
Vettoriale Stock PH indicator. Acidic, neutral, basic solutions. Color change of indicator paper Basic Indicator Solution The perceived color of an indicator solution is determined by the ratio of the concentrations of the two species in − and hin. This page assumes that you know about ph curves for all the. Indicators are substances whose solutions change color due to changes in ph. Only a small amount of indicator compound is. A ph less than 7. Basic Indicator Solution.
From ib2chemtasis.blogspot.com
IB2 Chemistry at TASIS Acid/ Base Indicators Basic Indicator Solution Ultimately, the ph value indicates how much h +. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. The perceived color of an indicator solution is determined by the ratio of the concentrations of the two species in − and hin. The quantity of. Basic Indicator Solution.
From sciencenotes.org
Universal Indicator Chart and Recipes Basic Indicator Solution The quantity of indicator in. Ultimately, the ph value indicates how much h +. Indicators are substances whose solutions change color due to changes in ph. A ph less than 7 indicates an acidic solution and a ph greater than 7 indicates a basic solution. This page assumes that you know about ph curves for all the. In more basic. Basic Indicator Solution.
From slidetodoc.com
Acids and Bases Properties of Acids and Bases Basic Indicator Solution In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Indicators are substances whose solutions change color due to changes in ph. The perceived color of an indicator solution is determined by the ratio of the concentrations of the two species in − and hin.. Basic Indicator Solution.
From www.compoundchem.com
The Colours & Chemistry of pH Indicators Compound Interest Basic Indicator Solution The perceived color of an indicator solution is determined by the ratio of the concentrations of the two species in − and hin. Indicators are substances whose solutions change color due to changes in ph. Only a small amount of indicator compound is. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m. Basic Indicator Solution.
From studylib.net
Acid/Base Indicator Solutions Basic Indicator Solution In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. A ph less than 7 indicates an acidic solution and a ph greater than 7 indicates a basic solution. The perceived color of an indicator solution is determined by the ratio of the concentrations of. Basic Indicator Solution.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID5741622 Basic Indicator Solution Only a small amount of indicator compound is. Indicators are substances whose solutions change color due to changes in ph. This page assumes that you know about ph curves for all the. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. The quantity of. Basic Indicator Solution.
From www.youtube.com
How to make a Phenolphthalein Indicator Solution (0.05wt) YouTube Basic Indicator Solution Ultimately, the ph value indicates how much h +. The perceived color of an indicator solution is determined by the ratio of the concentrations of the two species in − and hin. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Indicators are substances. Basic Indicator Solution.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Basic Indicator Solution The quantity of indicator in. The perceived color of an indicator solution is determined by the ratio of the concentrations of the two species in − and hin. Indicators are substances whose solutions change color due to changes in ph. Ultimately, the ph value indicates how much h +. A ph less than 7 indicates an acidic solution and a. Basic Indicator Solution.
From www.slideserve.com
PPT Solution Concentration PowerPoint Presentation, free download ID2145053 Basic Indicator Solution In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. A ph less than 7 indicates an acidic solution and a ph greater than 7 indicates a basic solution. Only a small amount of indicator compound is. Indicators are substances whose solutions change color due. Basic Indicator Solution.
From fphoto.photoshelter.com
science chemistry acid base indicator phenol red Fundamental Photographs The Art of Science Basic Indicator Solution The quantity of indicator in. Ultimately, the ph value indicates how much h +. The perceived color of an indicator solution is determined by the ratio of the concentrations of the two species in − and hin. A ph less than 7 indicates an acidic solution and a ph greater than 7 indicates a basic solution. Only a small amount. Basic Indicator Solution.
From study.com
Acidic, Basic & Neutral Solutions Overview, pH Scale & Uses Lesson Basic Indicator Solution This page assumes that you know about ph curves for all the. The quantity of indicator in. Only a small amount of indicator compound is. The perceived color of an indicator solution is determined by the ratio of the concentrations of the two species in − and hin. Ultimately, the ph value indicates how much h +. In more basic. Basic Indicator Solution.
From www.dreamstime.com
PH Paper Indicator with Acidic, Neutral and Basic Solutions Stock Illustration Illustration of Basic Indicator Solution The quantity of indicator in. Indicators are substances whose solutions change color due to changes in ph. Ultimately, the ph value indicates how much h +. A ph less than 7 indicates an acidic solution and a ph greater than 7 indicates a basic solution. This page assumes that you know about ph curves for all the. The perceived color. Basic Indicator Solution.
From stock.adobe.com
Vector illustration of Litmus pH paper indicators in chemical container. Litmus test blue and Basic Indicator Solution Indicators are substances whose solutions change color due to changes in ph. A ph less than 7 indicates an acidic solution and a ph greater than 7 indicates a basic solution. The quantity of indicator in. This page assumes that you know about ph curves for all the. In more basic solutions where the hydronium ion concentration is less than. Basic Indicator Solution.
From www.slideshare.net
Indicators Basic Indicator Solution A ph less than 7 indicates an acidic solution and a ph greater than 7 indicates a basic solution. Ultimately, the ph value indicates how much h +. Indicators are substances whose solutions change color due to changes in ph. The perceived color of an indicator solution is determined by the ratio of the concentrations of the two species in. Basic Indicator Solution.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Basic Indicator Solution The quantity of indicator in. This page assumes that you know about ph curves for all the. The perceived color of an indicator solution is determined by the ratio of the concentrations of the two species in − and hin. A ph less than 7 indicates an acidic solution and a ph greater than 7 indicates a basic solution. Ultimately,. Basic Indicator Solution.
From www.slideserve.com
PPT Properties of Acids PowerPoint Presentation, free download ID5368605 Basic Indicator Solution The perceived color of an indicator solution is determined by the ratio of the concentrations of the two species in − and hin. Only a small amount of indicator compound is. Indicators are substances whose solutions change color due to changes in ph. A ph less than 7 indicates an acidic solution and a ph greater than 7 indicates a. Basic Indicator Solution.
From www.slideserve.com
PPT AcidBase Equilibria PowerPoint Presentation, free download ID3196271 Basic Indicator Solution The perceived color of an indicator solution is determined by the ratio of the concentrations of the two species in − and hin. Indicators are substances whose solutions change color due to changes in ph. A ph less than 7 indicates an acidic solution and a ph greater than 7 indicates a basic solution. The quantity of indicator in. This. Basic Indicator Solution.
From benzmicroscope.com
Chemicals pH Indicator Solution Set (BZ0003) Benz Microscope Optics Center Basic Indicator Solution The quantity of indicator in. Indicators are substances whose solutions change color due to changes in ph. A ph less than 7 indicates an acidic solution and a ph greater than 7 indicates a basic solution. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or. Basic Indicator Solution.
From www.priyamstudycentre.com
Phenolphthalein Indicator, Solution, Uses Basic Indicator Solution Ultimately, the ph value indicates how much h +. The quantity of indicator in. Only a small amount of indicator compound is. The perceived color of an indicator solution is determined by the ratio of the concentrations of the two species in − and hin. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10. Basic Indicator Solution.
From www.bbc.co.uk
Indicators and the pH scale Acids, alkalis and the pH scale 3rd level Science Revision BBC Basic Indicator Solution The quantity of indicator in. Indicators are substances whose solutions change color due to changes in ph. A ph less than 7 indicates an acidic solution and a ph greater than 7 indicates a basic solution. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or. Basic Indicator Solution.
From www.slideserve.com
PPT Chapter 18 Acids, Bases & Salts PowerPoint Presentation, free download ID3041437 Basic Indicator Solution A ph less than 7 indicates an acidic solution and a ph greater than 7 indicates a basic solution. The quantity of indicator in. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Ultimately, the ph value indicates how much h +. This page. Basic Indicator Solution.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Basic Indicator Solution Indicators are substances whose solutions change color due to changes in ph. The perceived color of an indicator solution is determined by the ratio of the concentrations of the two species in − and hin. A ph less than 7 indicates an acidic solution and a ph greater than 7 indicates a basic solution. Only a small amount of indicator. Basic Indicator Solution.
From iteachly.com
Acid and Base Indicators Lab Activity ⋆ Basic Indicator Solution Ultimately, the ph value indicates how much h +. The quantity of indicator in. This page assumes that you know about ph curves for all the. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. The perceived color of an indicator solution is determined. Basic Indicator Solution.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Basic Indicator Solution The perceived color of an indicator solution is determined by the ratio of the concentrations of the two species in − and hin. Indicators are substances whose solutions change color due to changes in ph. A ph less than 7 indicates an acidic solution and a ph greater than 7 indicates a basic solution. The quantity of indicator in. This. Basic Indicator Solution.
From chemistry-acidsandbases.weebly.com
pH Scale and pH Indicators Basic Indicator Solution Only a small amount of indicator compound is. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Indicators are substances whose solutions change color due to changes in ph. This page assumes that you know about ph curves for all the. Ultimately, the ph. Basic Indicator Solution.