Standard Enthalpy Of Formation Ib Chem . Reaction enthapy may be determined directly by experiment, or by using other thermodynamic data such as enthalpies of formation, or bond. To compare the changes in enthalpy between reactions, all thermodynamic measurements are made under standard. The standard enthalpy of formation (∆hºf) is the enthalpy change that results when one mole of a compound is formed from its elements in their standard states (298ºk and. The lattice enthalpy (δh lat ꝋ) is defined as the standard enthalpy change that occurs on the formation of 1 mole of gaseous. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The energy is given per mole of substance. The enthalpy of formation of an element in its standard state is zero by this definition. The standard enthalpy change of formation refers to the energy shift occurring when one mole of a substance is assembled.
from www.numerade.com
The standard enthalpy of formation (∆hºf) is the enthalpy change that results when one mole of a compound is formed from its elements in their standard states (298ºk and. The lattice enthalpy (δh lat ꝋ) is defined as the standard enthalpy change that occurs on the formation of 1 mole of gaseous. Reaction enthapy may be determined directly by experiment, or by using other thermodynamic data such as enthalpies of formation, or bond. The enthalpy of formation of an element in its standard state is zero by this definition. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy change of formation refers to the energy shift occurring when one mole of a substance is assembled. To compare the changes in enthalpy between reactions, all thermodynamic measurements are made under standard. The energy is given per mole of substance.
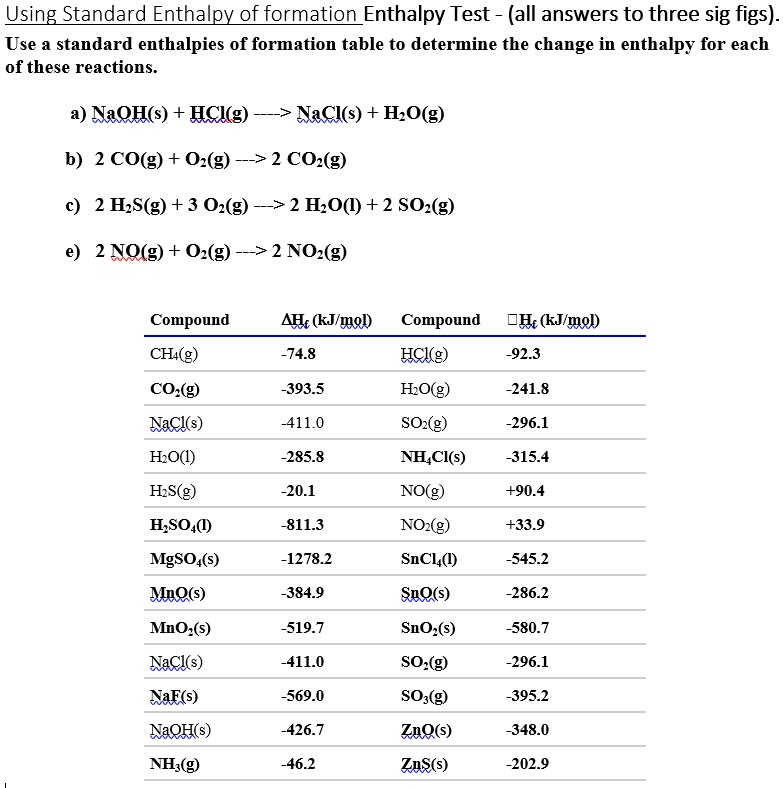
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all answers to three sig figs
Standard Enthalpy Of Formation Ib Chem To compare the changes in enthalpy between reactions, all thermodynamic measurements are made under standard. The standard enthalpy change of formation refers to the energy shift occurring when one mole of a substance is assembled. The enthalpy of formation of an element in its standard state is zero by this definition. The lattice enthalpy (δh lat ꝋ) is defined as the standard enthalpy change that occurs on the formation of 1 mole of gaseous. To compare the changes in enthalpy between reactions, all thermodynamic measurements are made under standard. The energy is given per mole of substance. Reaction enthapy may be determined directly by experiment, or by using other thermodynamic data such as enthalpies of formation, or bond. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation (∆hºf) is the enthalpy change that results when one mole of a compound is formed from its elements in their standard states (298ºk and.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies of Formation YouTube Standard Enthalpy Of Formation Ib Chem Reaction enthapy may be determined directly by experiment, or by using other thermodynamic data such as enthalpies of formation, or bond. The enthalpy of formation of an element in its standard state is zero by this definition. To compare the changes in enthalpy between reactions, all thermodynamic measurements are made under standard. The standard enthalpy change of formation refers to. Standard Enthalpy Of Formation Ib Chem.
From www.slideserve.com
PPT IB DP1 Chemistry Energetics PowerPoint Presentation, free download ID2020350 Standard Enthalpy Of Formation Ib Chem The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The enthalpy of formation of an element in its standard state is zero by this definition. The energy is given per mole of substance. Reaction enthapy may be determined directly by experiment, or by using other. Standard Enthalpy Of Formation Ib Chem.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Formation Ib Chem The standard enthalpy of formation (∆hºf) is the enthalpy change that results when one mole of a compound is formed from its elements in their standard states (298ºk and. The standard enthalpy change of formation refers to the energy shift occurring when one mole of a substance is assembled. To compare the changes in enthalpy between reactions, all thermodynamic measurements. Standard Enthalpy Of Formation Ib Chem.
From www.tessshebaylo.com
Balance The Following Chemical Equation And Calculate Standard Enthalpy Tessshebaylo Standard Enthalpy Of Formation Ib Chem The standard enthalpy of formation (∆hºf) is the enthalpy change that results when one mole of a compound is formed from its elements in their standard states (298ºk and. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. To compare the changes in enthalpy between. Standard Enthalpy Of Formation Ib Chem.
From www.chegg.com
Solved standard enthalpy of formation chem 1211 lab Standard Enthalpy Of Formation Ib Chem The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation (∆hºf) is the enthalpy change that results when one mole of a compound is formed from its elements in their standard states (298ºk and. Reaction enthapy may be determined directly by. Standard Enthalpy Of Formation Ib Chem.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Chemistry 2e 5.3 YouTube Standard Enthalpy Of Formation Ib Chem The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation (∆hºf) is the enthalpy change that results when one mole of a compound is formed from its elements in their standard states (298ºk and. To compare the changes in enthalpy between. Standard Enthalpy Of Formation Ib Chem.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation Ib Chem The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. To compare the changes in enthalpy between reactions, all thermodynamic measurements are made under standard. The standard enthalpy change of formation refers to the energy shift occurring when one mole of a substance is assembled. The. Standard Enthalpy Of Formation Ib Chem.
From mungfali.com
Standard Enthalpy Change Equation Standard Enthalpy Of Formation Ib Chem The lattice enthalpy (δh lat ꝋ) is defined as the standard enthalpy change that occurs on the formation of 1 mole of gaseous. The standard enthalpy change of formation refers to the energy shift occurring when one mole of a substance is assembled. The enthalpy of formation of an element in its standard state is zero by this definition. To. Standard Enthalpy Of Formation Ib Chem.
From mavink.com
Enthalpy Of Formation Equation Standard Enthalpy Of Formation Ib Chem The enthalpy of formation of an element in its standard state is zero by this definition. The energy is given per mole of substance. Reaction enthapy may be determined directly by experiment, or by using other thermodynamic data such as enthalpies of formation, or bond. To compare the changes in enthalpy between reactions, all thermodynamic measurements are made under standard.. Standard Enthalpy Of Formation Ib Chem.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Ib Chem Reaction enthapy may be determined directly by experiment, or by using other thermodynamic data such as enthalpies of formation, or bond. The standard enthalpy change of formation refers to the energy shift occurring when one mole of a substance is assembled. The energy is given per mole of substance. To compare the changes in enthalpy between reactions, all thermodynamic measurements. Standard Enthalpy Of Formation Ib Chem.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Ib Chem The lattice enthalpy (δh lat ꝋ) is defined as the standard enthalpy change that occurs on the formation of 1 mole of gaseous. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation (∆hºf) is the enthalpy change that results when. Standard Enthalpy Of Formation Ib Chem.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Ib Chem The lattice enthalpy (δh lat ꝋ) is defined as the standard enthalpy change that occurs on the formation of 1 mole of gaseous. Reaction enthapy may be determined directly by experiment, or by using other thermodynamic data such as enthalpies of formation, or bond. To compare the changes in enthalpy between reactions, all thermodynamic measurements are made under standard. The. Standard Enthalpy Of Formation Ib Chem.
From www.youtube.com
Hess' Law (Enthalpy Changes) IB Chemistry Revision Course YouTube Standard Enthalpy Of Formation Ib Chem The standard enthalpy change of formation refers to the energy shift occurring when one mole of a substance is assembled. The standard enthalpy of formation (∆hºf) is the enthalpy change that results when one mole of a compound is formed from its elements in their standard states (298ºk and. The lattice enthalpy (δh lat ꝋ) is defined as the standard. Standard Enthalpy Of Formation Ib Chem.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Ib Chem The standard enthalpy of formation (∆hºf) is the enthalpy change that results when one mole of a compound is formed from its elements in their standard states (298ºk and. The energy is given per mole of substance. The enthalpy of formation of an element in its standard state is zero by this definition. The standard enthalpy of formation is a. Standard Enthalpy Of Formation Ib Chem.
From www.scribd.com
Standard Enthalpy of Formation PDF Solvation Chemical Process Engineering Standard Enthalpy Of Formation Ib Chem The standard enthalpy change of formation refers to the energy shift occurring when one mole of a substance is assembled. The energy is given per mole of substance. Reaction enthapy may be determined directly by experiment, or by using other thermodynamic data such as enthalpies of formation, or bond. The enthalpy of formation of an element in its standard state. Standard Enthalpy Of Formation Ib Chem.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Enthalpy Of Formation Ib Chem The enthalpy of formation of an element in its standard state is zero by this definition. To compare the changes in enthalpy between reactions, all thermodynamic measurements are made under standard. The energy is given per mole of substance. The lattice enthalpy (δh lat ꝋ) is defined as the standard enthalpy change that occurs on the formation of 1 mole. Standard Enthalpy Of Formation Ib Chem.
From studylib.net
Standard Enthalpy of Formation Standard Enthalpy Of Formation Ib Chem The enthalpy of formation of an element in its standard state is zero by this definition. The standard enthalpy of formation (∆hºf) is the enthalpy change that results when one mole of a compound is formed from its elements in their standard states (298ºk and. To compare the changes in enthalpy between reactions, all thermodynamic measurements are made under standard.. Standard Enthalpy Of Formation Ib Chem.
From www.slideshare.net
IB Chemistry on Bond Enthalpy, Enthalpy formation, combustion and atomization PPT Standard Enthalpy Of Formation Ib Chem The lattice enthalpy (δh lat ꝋ) is defined as the standard enthalpy change that occurs on the formation of 1 mole of gaseous. The standard enthalpy of formation (∆hºf) is the enthalpy change that results when one mole of a compound is formed from its elements in their standard states (298ºk and. The standard enthalpy change of formation refers to. Standard Enthalpy Of Formation Ib Chem.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Ib Chem Reaction enthapy may be determined directly by experiment, or by using other thermodynamic data such as enthalpies of formation, or bond. The enthalpy of formation of an element in its standard state is zero by this definition. To compare the changes in enthalpy between reactions, all thermodynamic measurements are made under standard. The standard enthalpy of formation is a measure. Standard Enthalpy Of Formation Ib Chem.
From www.youtube.com
5.1 Define Standard State, Enthalpy change of formation and combustion [SL IB Chemistry] YouTube Standard Enthalpy Of Formation Ib Chem The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Reaction enthapy may be determined directly by experiment, or by using other thermodynamic data such as enthalpies of formation, or bond. To compare the changes in enthalpy between reactions, all thermodynamic measurements are made under standard.. Standard Enthalpy Of Formation Ib Chem.
From www.madebyteachers.com
Standard Molar Enthalpy of Formation Calculations A Chemistry Worksheet Made By Teachers Standard Enthalpy Of Formation Ib Chem Reaction enthapy may be determined directly by experiment, or by using other thermodynamic data such as enthalpies of formation, or bond. The energy is given per mole of substance. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy change of formation refers. Standard Enthalpy Of Formation Ib Chem.
From www.chemistryspace.com
Standard Enthalpy of Formation Standard Enthalpy Of Formation Ib Chem The lattice enthalpy (δh lat ꝋ) is defined as the standard enthalpy change that occurs on the formation of 1 mole of gaseous. Reaction enthapy may be determined directly by experiment, or by using other thermodynamic data such as enthalpies of formation, or bond. The standard enthalpy change of formation refers to the energy shift occurring when one mole of. Standard Enthalpy Of Formation Ib Chem.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Ib Chem The lattice enthalpy (δh lat ꝋ) is defined as the standard enthalpy change that occurs on the formation of 1 mole of gaseous. Reaction enthapy may be determined directly by experiment, or by using other thermodynamic data such as enthalpies of formation, or bond. The standard enthalpy of formation is a measure of the energy released or consumed when one. Standard Enthalpy Of Formation Ib Chem.
From www.youtube.com
Standard Enthalpy of Formation 🔴 Chemistry for Class 11 YouTube Standard Enthalpy Of Formation Ib Chem The energy is given per mole of substance. The enthalpy of formation of an element in its standard state is zero by this definition. The lattice enthalpy (δh lat ꝋ) is defined as the standard enthalpy change that occurs on the formation of 1 mole of gaseous. The standard enthalpy change of formation refers to the energy shift occurring when. Standard Enthalpy Of Formation Ib Chem.
From rayb78.github.io
Heat Of Formation Chart Standard Enthalpy Of Formation Ib Chem The enthalpy of formation of an element in its standard state is zero by this definition. Reaction enthapy may be determined directly by experiment, or by using other thermodynamic data such as enthalpies of formation, or bond. The standard enthalpy of formation (∆hºf) is the enthalpy change that results when one mole of a compound is formed from its elements. Standard Enthalpy Of Formation Ib Chem.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all answers to three sig figs Standard Enthalpy Of Formation Ib Chem The lattice enthalpy (δh lat ꝋ) is defined as the standard enthalpy change that occurs on the formation of 1 mole of gaseous. The enthalpy of formation of an element in its standard state is zero by this definition. Reaction enthapy may be determined directly by experiment, or by using other thermodynamic data such as enthalpies of formation, or bond.. Standard Enthalpy Of Formation Ib Chem.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF Standard Enthalpy Of Formation Ib Chem The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The enthalpy of formation of an element in its standard state is zero by this definition. The lattice enthalpy (δh lat ꝋ) is defined as the standard enthalpy change that occurs on the formation of 1. Standard Enthalpy Of Formation Ib Chem.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free download ID4097208 Standard Enthalpy Of Formation Ib Chem The lattice enthalpy (δh lat ꝋ) is defined as the standard enthalpy change that occurs on the formation of 1 mole of gaseous. The standard enthalpy of formation (∆hºf) is the enthalpy change that results when one mole of a compound is formed from its elements in their standard states (298ºk and. The standard enthalpy change of formation refers to. Standard Enthalpy Of Formation Ib Chem.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Ib Chem To compare the changes in enthalpy between reactions, all thermodynamic measurements are made under standard. The enthalpy of formation of an element in its standard state is zero by this definition. The energy is given per mole of substance. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is. Standard Enthalpy Of Formation Ib Chem.
From www.blendspace.com
Dp Y2 Biochem Option Lesson 4 Biochemistry & The Environment Lessons Blendspace Standard Enthalpy Of Formation Ib Chem To compare the changes in enthalpy between reactions, all thermodynamic measurements are made under standard. The standard enthalpy change of formation refers to the energy shift occurring when one mole of a substance is assembled. The enthalpy of formation of an element in its standard state is zero by this definition. The energy is given per mole of substance. The. Standard Enthalpy Of Formation Ib Chem.
From www.nagwa.com
Question Video Calculating the Standard Enthalpy of Formation for Heptane Using Standard Standard Enthalpy Of Formation Ib Chem The standard enthalpy change of formation refers to the energy shift occurring when one mole of a substance is assembled. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation (∆hºf) is the enthalpy change that results when one mole of. Standard Enthalpy Of Formation Ib Chem.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation Ib Chem The energy is given per mole of substance. The standard enthalpy of formation (∆hºf) is the enthalpy change that results when one mole of a compound is formed from its elements in their standard states (298ºk and. The enthalpy of formation of an element in its standard state is zero by this definition. The standard enthalpy change of formation refers. Standard Enthalpy Of Formation Ib Chem.
From studylib.net
Standard Enthalpy of Formation Standard Enthalpy Of Formation Ib Chem The standard enthalpy of formation (∆hºf) is the enthalpy change that results when one mole of a compound is formed from its elements in their standard states (298ºk and. Reaction enthapy may be determined directly by experiment, or by using other thermodynamic data such as enthalpies of formation, or bond. The energy is given per mole of substance. The enthalpy. Standard Enthalpy Of Formation Ib Chem.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Change YouTube Standard Enthalpy Of Formation Ib Chem The energy is given per mole of substance. To compare the changes in enthalpy between reactions, all thermodynamic measurements are made under standard. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The enthalpy of formation of an element in its standard state is zero. Standard Enthalpy Of Formation Ib Chem.
From courses.lumenlearning.com
9.3 Enthalpy General College Chemistry I Standard Enthalpy Of Formation Ib Chem To compare the changes in enthalpy between reactions, all thermodynamic measurements are made under standard. The standard enthalpy change of formation refers to the energy shift occurring when one mole of a substance is assembled. Reaction enthapy may be determined directly by experiment, or by using other thermodynamic data such as enthalpies of formation, or bond. The lattice enthalpy (δh. Standard Enthalpy Of Formation Ib Chem.