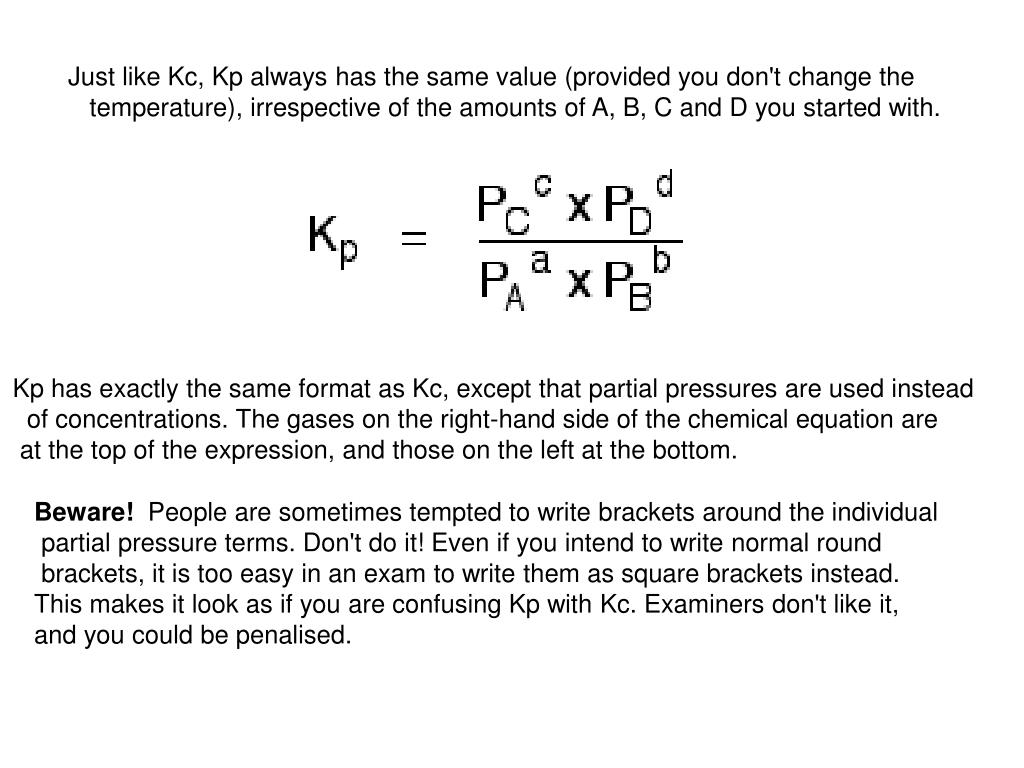
Does The Value Of Kc Change With Temperature . the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you started with. after asking around i have come to the conclusion that a reaction has many kc values depending on temperature , but then i. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the. learn how to calculate the equilibrium constant kc from the expression for chemical equilibrium. Find out how kc depends on temperature,. learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on. The units for k c can. Only temperature changes the value of k c. learn how equilibrium constants are not affected by changing concentrations or pressures, but only by changing. changing the concentrations of the reactants and products doesn’t change the value of k c for a reaction. revision notes on 1.8.5 changes which affect the equilibrium for the aqa a level chemistry syllabus, written by the chemistry experts at.
from www.slideserve.com
learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on. The units for k c can. changing the concentrations of the reactants and products doesn’t change the value of k c for a reaction. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you started with. after asking around i have come to the conclusion that a reaction has many kc values depending on temperature , but then i. Find out how kc depends on temperature,. revision notes on 1.8.5 changes which affect the equilibrium for the aqa a level chemistry syllabus, written by the chemistry experts at. learn how to calculate the equilibrium constant kc from the expression for chemical equilibrium. learn how equilibrium constants are not affected by changing concentrations or pressures, but only by changing. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the.
PPT EQUILIBRIUM CONSTANTS Kp PowerPoint Presentation, free download
Does The Value Of Kc Change With Temperature an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the. learn how equilibrium constants are not affected by changing concentrations or pressures, but only by changing. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you started with. revision notes on 1.8.5 changes which affect the equilibrium for the aqa a level chemistry syllabus, written by the chemistry experts at. learn how to calculate the equilibrium constant kc from the expression for chemical equilibrium. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the. Only temperature changes the value of k c. The units for k c can. changing the concentrations of the reactants and products doesn’t change the value of k c for a reaction. learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on. Find out how kc depends on temperature,. after asking around i have come to the conclusion that a reaction has many kc values depending on temperature , but then i.
From worksheetmediastanley.z21.web.core.windows.net
Equilibrium Constant Kc Formula Does The Value Of Kc Change With Temperature Only temperature changes the value of k c. learn how to calculate the equilibrium constant kc from the expression for chemical equilibrium. after asking around i have come to the conclusion that a reaction has many kc values depending on temperature , but then i. learn how to calculate kc and kp for chemical reactions involving gases. Does The Value Of Kc Change With Temperature.
From www.youtube.com
7.1 What Happens to Kc When the Equilibrium Equation Coefficients are Does The Value Of Kc Change With Temperature learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the. changing the concentrations of the reactants and products doesn’t change the value of k c for a reaction. . Does The Value Of Kc Change With Temperature.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Does The Value Of Kc Change With Temperature the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you started with. learn how equilibrium constants are not affected by changing concentrations or pressures, but only by changing. changing the concentrations of the reactants and products doesn’t change the value of k. Does The Value Of Kc Change With Temperature.
From haipernews.com
How To Calculate Equilibrium Constant At Different Temperatures Haiper Does The Value Of Kc Change With Temperature The units for k c can. Only temperature changes the value of k c. learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on. learn how to calculate the equilibrium constant kc from the expression for chemical equilibrium. Find out how kc depends on temperature,. revision notes on. Does The Value Of Kc Change With Temperature.
From mohammadferswoodward.blogspot.com
Calculate the Kc Value for the a protein Binding Reaction. Does The Value Of Kc Change With Temperature changing the concentrations of the reactants and products doesn’t change the value of k c for a reaction. learn how equilibrium constants are not affected by changing concentrations or pressures, but only by changing. after asking around i have come to the conclusion that a reaction has many kc values depending on temperature , but then i.. Does The Value Of Kc Change With Temperature.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Does The Value Of Kc Change With Temperature revision notes on 1.8.5 changes which affect the equilibrium for the aqa a level chemistry syllabus, written by the chemistry experts at. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the. Only temperature changes the value of k c. learn how equilibrium constants are not affected by. Does The Value Of Kc Change With Temperature.
From www.sarthaks.com
Rate constant vs temperature graph looks like If the activation energy Does The Value Of Kc Change With Temperature the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you started with. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the. Find out how kc depends on temperature,. Only temperature changes the value. Does The Value Of Kc Change With Temperature.
From ashnaschemblog.blogspot.com
Ashna's Chemistry Blog Does The Value Of Kc Change With Temperature Only temperature changes the value of k c. revision notes on 1.8.5 changes which affect the equilibrium for the aqa a level chemistry syllabus, written by the chemistry experts at. learn how equilibrium constants are not affected by changing concentrations or pressures, but only by changing. the equilibrium constant always has the same value (provided you don't. Does The Value Of Kc Change With Temperature.
From www.numerade.com
SOLVED predict the change in the equilibrium constant KC that would Does The Value Of Kc Change With Temperature learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the. learn how to calculate the equilibrium constant kc from the expression for chemical equilibrium. the equilibrium constant always. Does The Value Of Kc Change With Temperature.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Does The Value Of Kc Change With Temperature The units for k c can. learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on. after asking around i have come to the conclusion that a reaction has many kc values depending on temperature , but then i. learn how to calculate the equilibrium constant kc from. Does The Value Of Kc Change With Temperature.
From secondaryscience4all.wordpress.com
1.10 Equilibrium constant Kc for homogeneous systems (Equilibrium A2 Does The Value Of Kc Change With Temperature The units for k c can. learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the. after asking around i have come to the conclusion that a reaction has. Does The Value Of Kc Change With Temperature.
From www.homeworklib.com
What is the value for KC at this temperature? HomeworkLib Does The Value Of Kc Change With Temperature Only temperature changes the value of k c. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the. learn how to calculate the equilibrium constant kc from the expression for chemical equilibrium. revision notes on 1.8.5 changes which affect the equilibrium for the aqa a level chemistry syllabus,. Does The Value Of Kc Change With Temperature.
From www.youtube.com
The value of KC for the reaction 2A ⇌ B + C is 2 X 103. YouTube Does The Value Of Kc Change With Temperature Find out how kc depends on temperature,. Only temperature changes the value of k c. changing the concentrations of the reactants and products doesn’t change the value of k c for a reaction. learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on. learn how equilibrium constants are. Does The Value Of Kc Change With Temperature.
From haipernews.com
How To Calculate Kc Using Kp Haiper Does The Value Of Kc Change With Temperature changing the concentrations of the reactants and products doesn’t change the value of k c for a reaction. Only temperature changes the value of k c. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you started with. an equilibrium constant calculated. Does The Value Of Kc Change With Temperature.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID6271487 Does The Value Of Kc Change With Temperature learn how equilibrium constants are not affected by changing concentrations or pressures, but only by changing. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c. Does The Value Of Kc Change With Temperature.
From tfvp.org
Why Is Kc Affected By Temperature Unveiling The Thermodynamic Mystery Does The Value Of Kc Change With Temperature learn how to calculate the equilibrium constant kc from the expression for chemical equilibrium. Only temperature changes the value of k c. Find out how kc depends on temperature,. learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on. revision notes on 1.8.5 changes which affect the equilibrium. Does The Value Of Kc Change With Temperature.
From www.slideserve.com
PPT Factors that Affect Equilibrium PowerPoint Presentation, free Does The Value Of Kc Change With Temperature learn how to calculate the equilibrium constant kc from the expression for chemical equilibrium. changing the concentrations of the reactants and products doesn’t change the value of k c for a reaction. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you. Does The Value Of Kc Change With Temperature.
From haipernews.com
How To Calculate Kc When Given Kp And Temperature Haiper Does The Value Of Kc Change With Temperature after asking around i have come to the conclusion that a reaction has many kc values depending on temperature , but then i. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the. learn how equilibrium constants are not affected by changing concentrations or pressures, but only by. Does The Value Of Kc Change With Temperature.
From haipernews.com
How To Calculate Kc Given Concentration Haiper Does The Value Of Kc Change With Temperature learn how equilibrium constants are not affected by changing concentrations or pressures, but only by changing. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you started with. The units for k c can. changing the concentrations of the reactants and products. Does The Value Of Kc Change With Temperature.
From www.slideserve.com
PPT Equilibrium and Stability Constants PowerPoint Presentation, free Does The Value Of Kc Change With Temperature after asking around i have come to the conclusion that a reaction has many kc values depending on temperature , but then i. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the. changing the concentrations of the reactants and products doesn’t change the value of k c. Does The Value Of Kc Change With Temperature.
From www.youtube.com
19 Only temperature affects Kc YouTube Does The Value Of Kc Change With Temperature an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the. changing the concentrations of the reactants and products doesn’t change the value of k c for a reaction. learn how to calculate the equilibrium constant kc from the expression for chemical equilibrium. revision notes on 1.8.5 changes. Does The Value Of Kc Change With Temperature.
From www.chem.fsu.edu
Colloids Does The Value Of Kc Change With Temperature Only temperature changes the value of k c. Find out how kc depends on temperature,. The units for k c can. learn how to calculate the equilibrium constant kc from the expression for chemical equilibrium. revision notes on 1.8.5 changes which affect the equilibrium for the aqa a level chemistry syllabus, written by the chemistry experts at. . Does The Value Of Kc Change With Temperature.
From www.chegg.com
Solved Under what conditions are the values of Kc and Kp for Does The Value Of Kc Change With Temperature learn how equilibrium constants are not affected by changing concentrations or pressures, but only by changing. changing the concentrations of the reactants and products doesn’t change the value of k c for a reaction. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the. The units for k. Does The Value Of Kc Change With Temperature.
From www.youtube.com
CHEM 201 Calculating Equilibrium Concentrations from K and Initial Does The Value Of Kc Change With Temperature learn how to calculate the equilibrium constant kc from the expression for chemical equilibrium. Only temperature changes the value of k c. Find out how kc depends on temperature,. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you started with. learn. Does The Value Of Kc Change With Temperature.
From www.slideserve.com
PPT Factors that Affect Equilibrium PowerPoint Presentation, free Does The Value Of Kc Change With Temperature learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on. Find out how kc depends on temperature,. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you started with. after asking around i. Does The Value Of Kc Change With Temperature.
From www.youtube.com
Chemical Equilibrium Constant K Ice Tables Kp and Kc YouTube Does The Value Of Kc Change With Temperature changing the concentrations of the reactants and products doesn’t change the value of k c for a reaction. learn how to calculate the equilibrium constant kc from the expression for chemical equilibrium. learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on. revision notes on 1.8.5 changes. Does The Value Of Kc Change With Temperature.
From www.slideserve.com
PPT The Equilibrium Constant, K, and The Reaction Quotient, Q Does The Value Of Kc Change With Temperature revision notes on 1.8.5 changes which affect the equilibrium for the aqa a level chemistry syllabus, written by the chemistry experts at. Only temperature changes the value of k c. Find out how kc depends on temperature,. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b,. Does The Value Of Kc Change With Temperature.
From www.slideserve.com
PPT EQUILIBRIUM CONSTANTS Kp PowerPoint Presentation, free download Does The Value Of Kc Change With Temperature revision notes on 1.8.5 changes which affect the equilibrium for the aqa a level chemistry syllabus, written by the chemistry experts at. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the. Only temperature changes the value of k c. after asking around i have come to the. Does The Value Of Kc Change With Temperature.
From www.slideserve.com
PPT Chapter 9 Chemical Equilibrium PowerPoint Presentation, free Does The Value Of Kc Change With Temperature after asking around i have come to the conclusion that a reaction has many kc values depending on temperature , but then i. The units for k c can. Only temperature changes the value of k c. revision notes on 1.8.5 changes which affect the equilibrium for the aqa a level chemistry syllabus, written by the chemistry experts. Does The Value Of Kc Change With Temperature.
From socratic.org
How do I relate equilibrium constants to temperature change to find the Does The Value Of Kc Change With Temperature an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the. learn how equilibrium constants are not affected by changing concentrations or pressures, but only by changing. Find out how kc depends on temperature,. after asking around i have come to the conclusion that a reaction has many kc. Does The Value Of Kc Change With Temperature.
From www.showme.com
Qc and Kc in equilibrium changes Science, Chemistry, Physical Does The Value Of Kc Change With Temperature an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the. learn how to calculate the equilibrium constant kc from the expression for chemical equilibrium. The units for k c can. after asking around i have come to the conclusion that a reaction has many kc values depending on. Does The Value Of Kc Change With Temperature.
From www.youtube.com
Equilibrium Constant from Concentration 2 (Example) YouTube Does The Value Of Kc Change With Temperature an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the. after asking around i have come to the conclusion that a reaction has many kc values depending on temperature , but then i. changing the concentrations of the reactants and products doesn’t change the value of k c. Does The Value Of Kc Change With Temperature.
From studyschlafkurdo.z21.web.core.windows.net
Chemical Equilibrium Formula Sheet Does The Value Of Kc Change With Temperature changing the concentrations of the reactants and products doesn’t change the value of k c for a reaction. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you started with. learn how to calculate the equilibrium constant kc from the expression for. Does The Value Of Kc Change With Temperature.
From www.coursehero.com
[Solved] . Example 2 The equilibrium value of Kc for the reaction Does The Value Of Kc Change With Temperature Only temperature changes the value of k c. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you started with. Find out how kc depends on temperature,. learn how to calculate the equilibrium constant kc from the expression for chemical equilibrium. learn. Does The Value Of Kc Change With Temperature.
From haipernews.com
How To Calculate Kc In Chemical Equilibrium Haiper Does The Value Of Kc Change With Temperature the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of a, b, c and d you started with. The units for k c can. learn how to calculate the equilibrium constant kc from the expression for chemical equilibrium. revision notes on 1.8.5 changes which affect the equilibrium for. Does The Value Of Kc Change With Temperature.