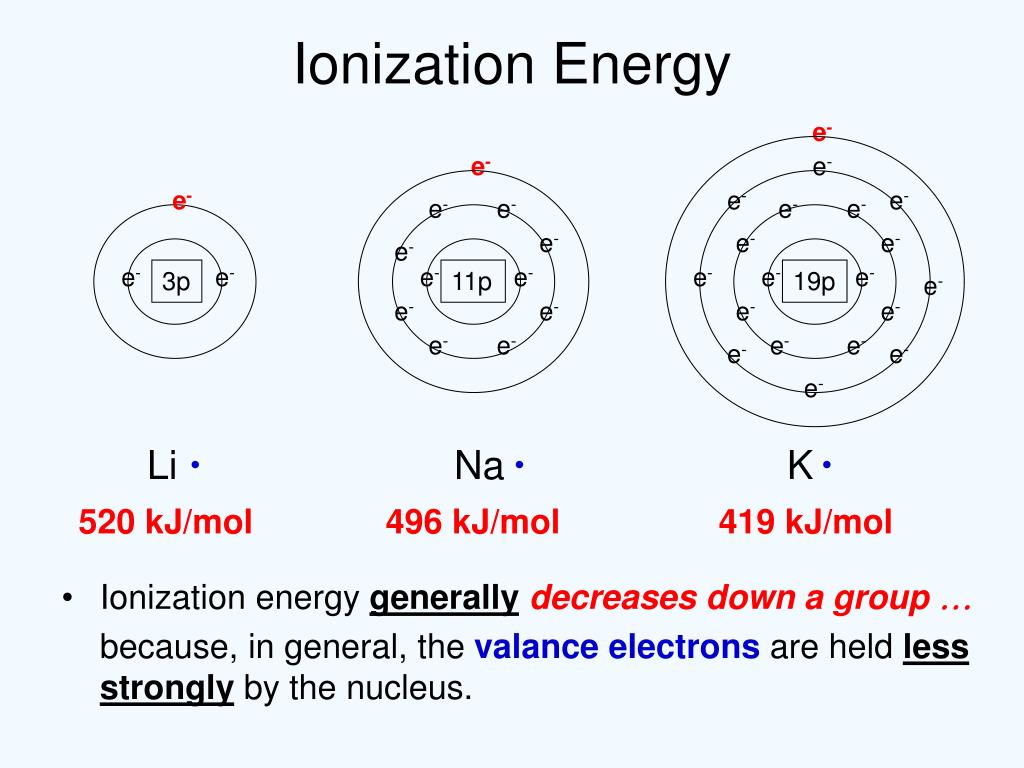
Does Boron Have A Higher Ionization Energy Than Fluorine . learn how to calculate the ionization energy of lithium and other elements using the general equation and. the first ionization energy of boron is smaller than beryllium, and the first ionization energy of oxygen is smaller than nitrogen. 123 rows these tables list values of molar ionization energies, measured in kj⋅mol −1. Explore the trends and exceptions in. learn how ionization energies measure the energy required to remove electrons from atoms and ions, and how they vary with. find the ionization energy of sodium, magnesium, phosphorus and chlorine in electron volts (ev) from this. This is the energy per mole necessary to. Explore the trends and patterns of ionization. learn how ionization energy is defined, measured, and related to the periodic properties of the elements. learn what ionization energy is, how it varies across the periodic table, and what factors affect its size.
from www.slideserve.com
learn how ionization energies measure the energy required to remove electrons from atoms and ions, and how they vary with. 123 rows these tables list values of molar ionization energies, measured in kj⋅mol −1. This is the energy per mole necessary to. learn what ionization energy is, how it varies across the periodic table, and what factors affect its size. the first ionization energy of boron is smaller than beryllium, and the first ionization energy of oxygen is smaller than nitrogen. learn how to calculate the ionization energy of lithium and other elements using the general equation and. Explore the trends and patterns of ionization. find the ionization energy of sodium, magnesium, phosphorus and chlorine in electron volts (ev) from this. Explore the trends and exceptions in. learn how ionization energy is defined, measured, and related to the periodic properties of the elements.
PPT Ionization Energy PowerPoint Presentation, free download ID6548427
Does Boron Have A Higher Ionization Energy Than Fluorine learn how ionization energy is defined, measured, and related to the periodic properties of the elements. the first ionization energy of boron is smaller than beryllium, and the first ionization energy of oxygen is smaller than nitrogen. This is the energy per mole necessary to. learn how to calculate the ionization energy of lithium and other elements using the general equation and. 123 rows these tables list values of molar ionization energies, measured in kj⋅mol −1. learn what ionization energy is, how it varies across the periodic table, and what factors affect its size. find the ionization energy of sodium, magnesium, phosphorus and chlorine in electron volts (ev) from this. Explore the trends and patterns of ionization. learn how ionization energies measure the energy required to remove electrons from atoms and ions, and how they vary with. learn how ionization energy is defined, measured, and related to the periodic properties of the elements. Explore the trends and exceptions in.
From www.britannica.com
Boron Properties, Uses, & Facts Britannica Does Boron Have A Higher Ionization Energy Than Fluorine learn how to calculate the ionization energy of lithium and other elements using the general equation and. the first ionization energy of boron is smaller than beryllium, and the first ionization energy of oxygen is smaller than nitrogen. Explore the trends and exceptions in. find the ionization energy of sodium, magnesium, phosphorus and chlorine in electron volts. Does Boron Have A Higher Ionization Energy Than Fluorine.
From exodkkfeq.blob.core.windows.net
Does Boron Have Ionization Energy at Roger Mull blog Does Boron Have A Higher Ionization Energy Than Fluorine find the ionization energy of sodium, magnesium, phosphorus and chlorine in electron volts (ev) from this. the first ionization energy of boron is smaller than beryllium, and the first ionization energy of oxygen is smaller than nitrogen. This is the energy per mole necessary to. 123 rows these tables list values of molar ionization energies, measured in. Does Boron Have A Higher Ionization Energy Than Fluorine.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Does Boron Have A Higher Ionization Energy Than Fluorine Explore the trends and patterns of ionization. the first ionization energy of boron is smaller than beryllium, and the first ionization energy of oxygen is smaller than nitrogen. learn how to calculate the ionization energy of lithium and other elements using the general equation and. learn how ionization energies measure the energy required to remove electrons from. Does Boron Have A Higher Ionization Energy Than Fluorine.
From periodictableguide.com
Periodic table with Ionization Energy Values (Labeled Image) Does Boron Have A Higher Ionization Energy Than Fluorine find the ionization energy of sodium, magnesium, phosphorus and chlorine in electron volts (ev) from this. the first ionization energy of boron is smaller than beryllium, and the first ionization energy of oxygen is smaller than nitrogen. Explore the trends and patterns of ionization. 123 rows these tables list values of molar ionization energies, measured in kj⋅mol. Does Boron Have A Higher Ionization Energy Than Fluorine.
From slideplayer.com
IONISATION ENERGY OBJECTIVES To define the term ‘ionisation energy’ To describe and explain the Does Boron Have A Higher Ionization Energy Than Fluorine learn how ionization energy is defined, measured, and related to the periodic properties of the elements. learn what ionization energy is, how it varies across the periodic table, and what factors affect its size. the first ionization energy of boron is smaller than beryllium, and the first ionization energy of oxygen is smaller than nitrogen. find. Does Boron Have A Higher Ionization Energy Than Fluorine.
From rubyowens.z21.web.core.windows.net
Ionization Energy Chart Periodic Table Does Boron Have A Higher Ionization Energy Than Fluorine Explore the trends and patterns of ionization. Explore the trends and exceptions in. learn how to calculate the ionization energy of lithium and other elements using the general equation and. the first ionization energy of boron is smaller than beryllium, and the first ionization energy of oxygen is smaller than nitrogen. learn what ionization energy is, how. Does Boron Have A Higher Ionization Energy Than Fluorine.
From therealgroupichem.weebly.com
Ionization energy Group i Chemistry(iiiescuro) Does Boron Have A Higher Ionization Energy Than Fluorine learn how ionization energy is defined, measured, and related to the periodic properties of the elements. 123 rows these tables list values of molar ionization energies, measured in kj⋅mol −1. Explore the trends and exceptions in. learn how to calculate the ionization energy of lithium and other elements using the general equation and. Explore the trends and. Does Boron Have A Higher Ionization Energy Than Fluorine.
From guidediagrampreconsume.z21.web.core.windows.net
Boron Electron Dot Diagram Does Boron Have A Higher Ionization Energy Than Fluorine Explore the trends and patterns of ionization. learn what ionization energy is, how it varies across the periodic table, and what factors affect its size. learn how to calculate the ionization energy of lithium and other elements using the general equation and. find the ionization energy of sodium, magnesium, phosphorus and chlorine in electron volts (ev) from. Does Boron Have A Higher Ionization Energy Than Fluorine.
From www.youtube.com
Periodic Trends Ionization Energy. YouTube Does Boron Have A Higher Ionization Energy Than Fluorine learn how ionization energy is defined, measured, and related to the periodic properties of the elements. 123 rows these tables list values of molar ionization energies, measured in kj⋅mol −1. the first ionization energy of boron is smaller than beryllium, and the first ionization energy of oxygen is smaller than nitrogen. find the ionization energy of. Does Boron Have A Higher Ionization Energy Than Fluorine.
From byjus.com
11.What is the correct order of increasing second ionisation energy in Lithium,Berilium,Boron Does Boron Have A Higher Ionization Energy Than Fluorine find the ionization energy of sodium, magnesium, phosphorus and chlorine in electron volts (ev) from this. This is the energy per mole necessary to. learn what ionization energy is, how it varies across the periodic table, and what factors affect its size. learn how to calculate the ionization energy of lithium and other elements using the general. Does Boron Have A Higher Ionization Energy Than Fluorine.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Does Boron Have A Higher Ionization Energy Than Fluorine 123 rows these tables list values of molar ionization energies, measured in kj⋅mol −1. Explore the trends and exceptions in. learn what ionization energy is, how it varies across the periodic table, and what factors affect its size. learn how ionization energies measure the energy required to remove electrons from atoms and ions, and how they vary. Does Boron Have A Higher Ionization Energy Than Fluorine.
From www.nuclear-power.com
Boron Electron Affinity Electronegativity Ionization Energy of Boron Does Boron Have A Higher Ionization Energy Than Fluorine find the ionization energy of sodium, magnesium, phosphorus and chlorine in electron volts (ev) from this. learn how ionization energy is defined, measured, and related to the periodic properties of the elements. learn how ionization energies measure the energy required to remove electrons from atoms and ions, and how they vary with. learn how to calculate. Does Boron Have A Higher Ionization Energy Than Fluorine.
From www.chem1.com
Periodic properties of the elements Does Boron Have A Higher Ionization Energy Than Fluorine This is the energy per mole necessary to. learn how ionization energy is defined, measured, and related to the periodic properties of the elements. learn how ionization energies measure the energy required to remove electrons from atoms and ions, and how they vary with. 123 rows these tables list values of molar ionization energies, measured in kj⋅mol. Does Boron Have A Higher Ionization Energy Than Fluorine.
From periodictable.me
How To Find The Electron Configuration For Boron Dynamic Periodic Table of Elements and Chemistry Does Boron Have A Higher Ionization Energy Than Fluorine learn how ionization energy is defined, measured, and related to the periodic properties of the elements. Explore the trends and exceptions in. learn how ionization energies measure the energy required to remove electrons from atoms and ions, and how they vary with. learn what ionization energy is, how it varies across the periodic table, and what factors. Does Boron Have A Higher Ionization Energy Than Fluorine.
From www.slideserve.com
PPT Chapter 6 Ionic Bonds and Some Main Group Chemistry PowerPoint Presentation ID5118935 Does Boron Have A Higher Ionization Energy Than Fluorine the first ionization energy of boron is smaller than beryllium, and the first ionization energy of oxygen is smaller than nitrogen. Explore the trends and patterns of ionization. Explore the trends and exceptions in. learn how ionization energy is defined, measured, and related to the periodic properties of the elements. find the ionization energy of sodium, magnesium,. Does Boron Have A Higher Ionization Energy Than Fluorine.
From www.youtube.com
Atomic Structure (Bohr Model) for Boron (B) YouTube Does Boron Have A Higher Ionization Energy Than Fluorine the first ionization energy of boron is smaller than beryllium, and the first ionization energy of oxygen is smaller than nitrogen. Explore the trends and patterns of ionization. Explore the trends and exceptions in. learn how ionization energies measure the energy required to remove electrons from atoms and ions, and how they vary with. learn what ionization. Does Boron Have A Higher Ionization Energy Than Fluorine.
From chem.libretexts.org
6.6 Ionization Energies Chemistry LibreTexts Does Boron Have A Higher Ionization Energy Than Fluorine 123 rows these tables list values of molar ionization energies, measured in kj⋅mol −1. learn how ionization energies measure the energy required to remove electrons from atoms and ions, and how they vary with. learn how ionization energy is defined, measured, and related to the periodic properties of the elements. find the ionization energy of sodium,. Does Boron Have A Higher Ionization Energy Than Fluorine.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID5631786 Does Boron Have A Higher Ionization Energy Than Fluorine This is the energy per mole necessary to. learn how ionization energy is defined, measured, and related to the periodic properties of the elements. the first ionization energy of boron is smaller than beryllium, and the first ionization energy of oxygen is smaller than nitrogen. Explore the trends and patterns of ionization. learn what ionization energy is,. Does Boron Have A Higher Ionization Energy Than Fluorine.
From www.slideserve.com
PPT Ionization Energy PowerPoint Presentation, free download ID6548427 Does Boron Have A Higher Ionization Energy Than Fluorine find the ionization energy of sodium, magnesium, phosphorus and chlorine in electron volts (ev) from this. learn how ionization energy is defined, measured, and related to the periodic properties of the elements. This is the energy per mole necessary to. learn what ionization energy is, how it varies across the periodic table, and what factors affect its. Does Boron Have A Higher Ionization Energy Than Fluorine.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Does Boron Have A Higher Ionization Energy Than Fluorine learn how to calculate the ionization energy of lithium and other elements using the general equation and. 123 rows these tables list values of molar ionization energies, measured in kj⋅mol −1. Explore the trends and exceptions in. find the ionization energy of sodium, magnesium, phosphorus and chlorine in electron volts (ev) from this. Explore the trends and. Does Boron Have A Higher Ionization Energy Than Fluorine.
From www.youtube.com
Electronegativity ¦ Chemistry of life ¦ Biology ¦ Khan Academy hd YouTube Does Boron Have A Higher Ionization Energy Than Fluorine 123 rows these tables list values of molar ionization energies, measured in kj⋅mol −1. learn what ionization energy is, how it varies across the periodic table, and what factors affect its size. Explore the trends and exceptions in. learn how to calculate the ionization energy of lithium and other elements using the general equation and. learn. Does Boron Have A Higher Ionization Energy Than Fluorine.
From exodkkfeq.blob.core.windows.net
Does Boron Have Ionization Energy at Roger Mull blog Does Boron Have A Higher Ionization Energy Than Fluorine This is the energy per mole necessary to. Explore the trends and exceptions in. learn how ionization energy is defined, measured, and related to the periodic properties of the elements. learn what ionization energy is, how it varies across the periodic table, and what factors affect its size. the first ionization energy of boron is smaller than. Does Boron Have A Higher Ionization Energy Than Fluorine.
From exodkkfeq.blob.core.windows.net
Does Boron Have Ionization Energy at Roger Mull blog Does Boron Have A Higher Ionization Energy Than Fluorine find the ionization energy of sodium, magnesium, phosphorus and chlorine in electron volts (ev) from this. the first ionization energy of boron is smaller than beryllium, and the first ionization energy of oxygen is smaller than nitrogen. learn how ionization energy is defined, measured, and related to the periodic properties of the elements. Explore the trends and. Does Boron Have A Higher Ionization Energy Than Fluorine.
From www.youtube.com
Statement1 Nitrogen atom has higher ionization energy than fluorine atom. Statement2 YouTube Does Boron Have A Higher Ionization Energy Than Fluorine learn how ionization energy is defined, measured, and related to the periodic properties of the elements. This is the energy per mole necessary to. 123 rows these tables list values of molar ionization energies, measured in kj⋅mol −1. learn how ionization energies measure the energy required to remove electrons from atoms and ions, and how they vary. Does Boron Have A Higher Ionization Energy Than Fluorine.
From www.geeksforgeeks.org
Ionization Energy Definition, Formulas, and Solved Examples Does Boron Have A Higher Ionization Energy Than Fluorine the first ionization energy of boron is smaller than beryllium, and the first ionization energy of oxygen is smaller than nitrogen. 123 rows these tables list values of molar ionization energies, measured in kj⋅mol −1. learn how ionization energies measure the energy required to remove electrons from atoms and ions, and how they vary with. learn. Does Boron Have A Higher Ionization Energy Than Fluorine.
From www.youtube.com
Successive Ionization Energies YouTube Does Boron Have A Higher Ionization Energy Than Fluorine the first ionization energy of boron is smaller than beryllium, and the first ionization energy of oxygen is smaller than nitrogen. learn how ionization energy is defined, measured, and related to the periodic properties of the elements. This is the energy per mole necessary to. learn how to calculate the ionization energy of lithium and other elements. Does Boron Have A Higher Ionization Energy Than Fluorine.
From chemistnotes.com
Periodic table Archives Chemistry Notes Does Boron Have A Higher Ionization Energy Than Fluorine Explore the trends and patterns of ionization. the first ionization energy of boron is smaller than beryllium, and the first ionization energy of oxygen is smaller than nitrogen. learn how ionization energies measure the energy required to remove electrons from atoms and ions, and how they vary with. learn what ionization energy is, how it varies across. Does Boron Have A Higher Ionization Energy Than Fluorine.
From www.slideserve.com
PPT Ionization Energy PowerPoint Presentation, free download ID5119239 Does Boron Have A Higher Ionization Energy Than Fluorine learn how ionization energy is defined, measured, and related to the periodic properties of the elements. Explore the trends and patterns of ionization. find the ionization energy of sodium, magnesium, phosphorus and chlorine in electron volts (ev) from this. the first ionization energy of boron is smaller than beryllium, and the first ionization energy of oxygen is. Does Boron Have A Higher Ionization Energy Than Fluorine.
From www.slideserve.com
PPT The Periodic Table & Its Properties Chapters 5 & 6 PowerPoint Presentation ID5525984 Does Boron Have A Higher Ionization Energy Than Fluorine 123 rows these tables list values of molar ionization energies, measured in kj⋅mol −1. Explore the trends and patterns of ionization. learn how ionization energy is defined, measured, and related to the periodic properties of the elements. Explore the trends and exceptions in. learn how to calculate the ionization energy of lithium and other elements using the. Does Boron Have A Higher Ionization Energy Than Fluorine.
From commons.wikimedia.org
FileIonization energy atomic size.svg Wikimedia Commons Does Boron Have A Higher Ionization Energy Than Fluorine learn how ionization energies measure the energy required to remove electrons from atoms and ions, and how they vary with. learn how to calculate the ionization energy of lithium and other elements using the general equation and. Explore the trends and patterns of ionization. This is the energy per mole necessary to. Explore the trends and exceptions in.. Does Boron Have A Higher Ionization Energy Than Fluorine.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Does Boron Have A Higher Ionization Energy Than Fluorine learn how ionization energy is defined, measured, and related to the periodic properties of the elements. learn what ionization energy is, how it varies across the periodic table, and what factors affect its size. the first ionization energy of boron is smaller than beryllium, and the first ionization energy of oxygen is smaller than nitrogen. This is. Does Boron Have A Higher Ionization Energy Than Fluorine.
From www.ck12.org
Periodic Trends in Ionization Energy CK12 Foundation Does Boron Have A Higher Ionization Energy Than Fluorine find the ionization energy of sodium, magnesium, phosphorus and chlorine in electron volts (ev) from this. Explore the trends and patterns of ionization. learn how to calculate the ionization energy of lithium and other elements using the general equation and. learn what ionization energy is, how it varies across the periodic table, and what factors affect its. Does Boron Have A Higher Ionization Energy Than Fluorine.
From learningmagictorres.z21.web.core.windows.net
How To Identify Ionization Energy Does Boron Have A Higher Ionization Energy Than Fluorine learn how ionization energies measure the energy required to remove electrons from atoms and ions, and how they vary with. find the ionization energy of sodium, magnesium, phosphorus and chlorine in electron volts (ev) from this. This is the energy per mole necessary to. learn how to calculate the ionization energy of lithium and other elements using. Does Boron Have A Higher Ionization Energy Than Fluorine.
From socratic.org
Why does fluorine have a higher ionization energy than bromine? Socratic Does Boron Have A Higher Ionization Energy Than Fluorine This is the energy per mole necessary to. 123 rows these tables list values of molar ionization energies, measured in kj⋅mol −1. learn how to calculate the ionization energy of lithium and other elements using the general equation and. learn what ionization energy is, how it varies across the periodic table, and what factors affect its size.. Does Boron Have A Higher Ionization Energy Than Fluorine.
From www.numerade.com
SOLVEDUsing an MO energylevel diagram, would you expect F2 to. have a lower or higher first Does Boron Have A Higher Ionization Energy Than Fluorine Explore the trends and exceptions in. Explore the trends and patterns of ionization. This is the energy per mole necessary to. learn how ionization energies measure the energy required to remove electrons from atoms and ions, and how they vary with. learn what ionization energy is, how it varies across the periodic table, and what factors affect its. Does Boron Have A Higher Ionization Energy Than Fluorine.