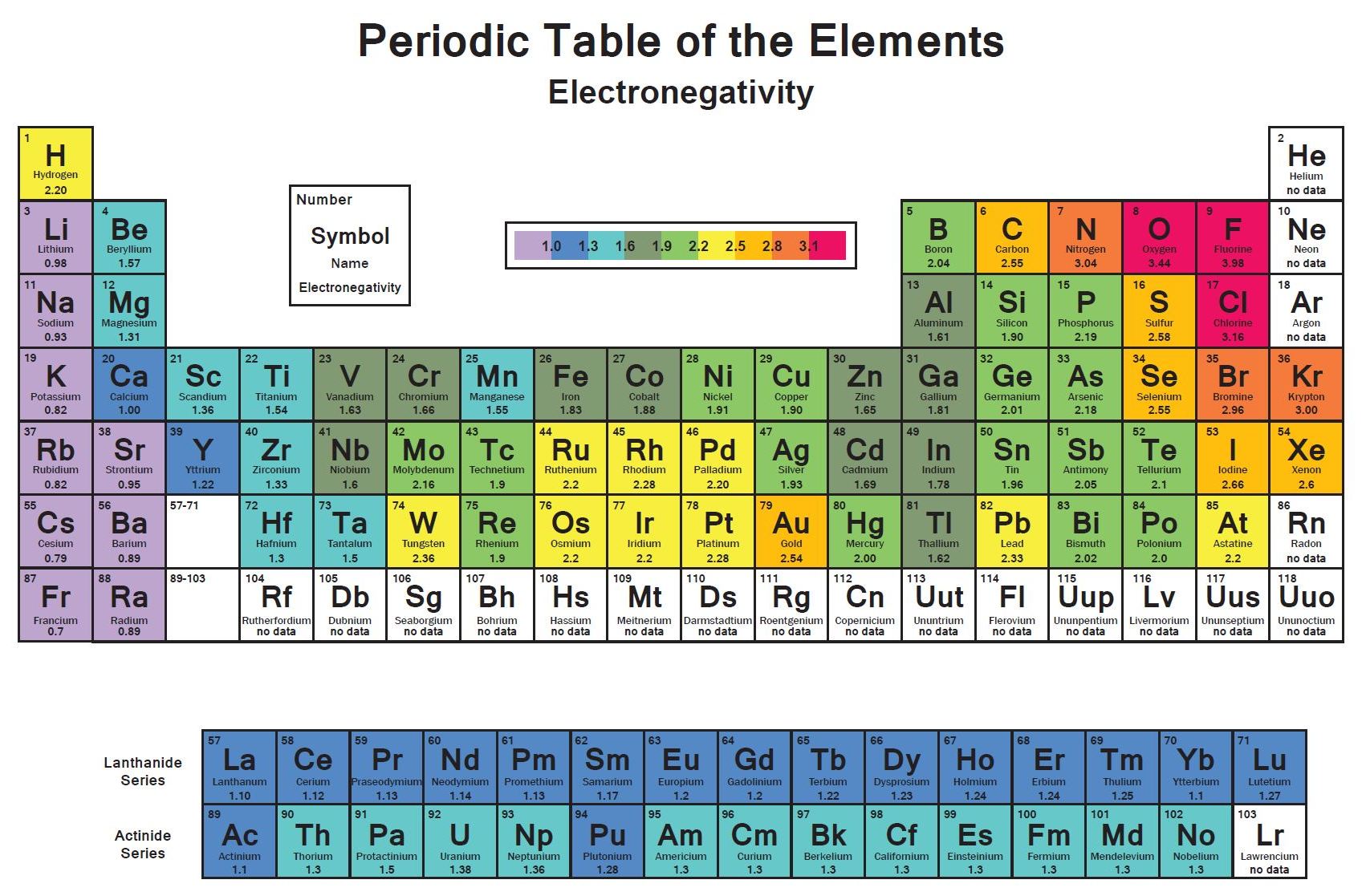
Electronegativity Of Chlorine Is Higher Than Sulphur . It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. examples include most covalent bonds. electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond. It can also be used to predict if the. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Because sr lies far to the left of. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). The suggested values are all. 3.16 is the electronegativity value of chlorine (cl). electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons.
from studylibrarygodward.z13.web.core.windows.net
119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. It can also be used to predict if the. The suggested values are all. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). examples include most covalent bonds. Because sr lies far to the left of. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond.
Difference In Electronegativity Worksheet
Electronegativity Of Chlorine Is Higher Than Sulphur electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. examples include most covalent bonds. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Because sr lies far to the left of. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). 103 rows electronegativity is not a uniquely defined property and may depend on the definition. The suggested values are all. It can also be used to predict if the. 3.16 is the electronegativity value of chlorine (cl).
From www.slideserve.com
PPT Section 6.5 “Polar Bonds and Intermolecular Forces” PowerPoint Electronegativity Of Chlorine Is Higher Than Sulphur It can also be used to predict if the. The suggested values are all. Because sr lies far to the left of. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. 119 rows electronegativity is. Electronegativity Of Chlorine Is Higher Than Sulphur.
From sciencenotes.org
List of Electronegativity Values of the Elements Electronegativity Of Chlorine Is Higher Than Sulphur Because sr lies far to the left of. examples include most covalent bonds. electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond. 3.16 is the electronegativity value of chlorine (cl). The suggested values are all. It belongs to the 7th group. Electronegativity Of Chlorine Is Higher Than Sulphur.
From studydiscretion.z13.web.core.windows.net
How To Determine Electronegativity Difference Electronegativity Of Chlorine Is Higher Than Sulphur It can also be used to predict if the. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. examples include most covalent bonds. Because sr lies far to the left of. 3.16 is the electronegativity value of chlorine (cl). electronegativity is a chemical property that measures how likely an. Electronegativity Of Chlorine Is Higher Than Sulphur.
From lessonlibmisweening.z22.web.core.windows.net
Lesson 7.5 Electronegativity And Polarity Electronegativity Of Chlorine Is Higher Than Sulphur It can also be used to predict if the. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. examples include most covalent bonds. 3.16 is the electronegativity value of chlorine (cl). electronegativity. Electronegativity Of Chlorine Is Higher Than Sulphur.
From www.thoughtco.com
Printable Periodic Table of the Elements Electronegativity Electronegativity Of Chlorine Is Higher Than Sulphur a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. 3.16 is the electronegativity value of chlorine (cl). It. Electronegativity Of Chlorine Is Higher Than Sulphur.
From mungfali.com
Electronegativity And Electronegativity Chart In Pdf 7C1 Electronegativity Of Chlorine Is Higher Than Sulphur a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). examples include most covalent bonds. It can also be used to predict if the. Because sr lies far to the left of. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It belongs to. Electronegativity Of Chlorine Is Higher Than Sulphur.
From learningschoolpistadasso.z22.web.core.windows.net
Electronegativity And Polarity Chart Electronegativity Of Chlorine Is Higher Than Sulphur Because sr lies far to the left of. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. It can also be used to predict if the. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). 3.16 is the electronegativity value of chlorine (cl). The suggested. Electronegativity Of Chlorine Is Higher Than Sulphur.
From sciencetrends.com
Electronegativity Chart Science Trends Electronegativity Of Chlorine Is Higher Than Sulphur examples include most covalent bonds. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond. It belongs to the 7th group and 2nd period on the periodic. Electronegativity Of Chlorine Is Higher Than Sulphur.
From mungfali.com
Electronegativity Trend Periodic Table Electronegativity Of Chlorine Is Higher Than Sulphur Because sr lies far to the left of. It can also be used to predict if the. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). examples include most covalent bonds. 103 rows. Electronegativity Of Chlorine Is Higher Than Sulphur.
From www.numerade.com
SOLVED Consider the molecules below Classify each bond as an ionic Electronegativity Of Chlorine Is Higher Than Sulphur It can also be used to predict if the. examples include most covalent bonds. The suggested values are all. 3.16 is the electronegativity value of chlorine (cl). It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. electronegativity is a chemical property that measures how likely an atom is to. Electronegativity Of Chlorine Is Higher Than Sulphur.
From neurotext.library.stonybrook.edu
Cellular Neurophysiology Electronegativity Of Chlorine Is Higher Than Sulphur 3.16 is the electronegativity value of chlorine (cl). a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). 103 rows electronegativity is not a uniquely defined property and may depend on the definition. examples include most covalent bonds. electronegativity is a measure of the tendency of an atom to attract. Electronegativity Of Chlorine Is Higher Than Sulphur.
From studylibrarygodward.z13.web.core.windows.net
Difference In Electronegativity Worksheet Electronegativity Of Chlorine Is Higher Than Sulphur It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond. It can also be used to predict if the. 3.16 is the electronegativity value of chlorine. Electronegativity Of Chlorine Is Higher Than Sulphur.
From mavink.com
Element Electronegativity Chart Electronegativity Of Chlorine Is Higher Than Sulphur It can also be used to predict if the. electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. examples include most covalent bonds. . Electronegativity Of Chlorine Is Higher Than Sulphur.
From learningschoolpistadasso.z22.web.core.windows.net
Electronegativity And Bond Polarity Chart Electronegativity Of Chlorine Is Higher Than Sulphur a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It can also be used to predict if the. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens.. Electronegativity Of Chlorine Is Higher Than Sulphur.
From www.toppr.com
Electronegativity of chlorine is three. Electron affinity of chlorine Electronegativity Of Chlorine Is Higher Than Sulphur It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. It can also be used to predict if the. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. examples include most covalent bonds. a electronegativity increases from lower left to upper right. Electronegativity Of Chlorine Is Higher Than Sulphur.
From exocoyzqy.blob.core.windows.net
Does Chlorine Have A High Electronegativity at Gordon Maxwell blog Electronegativity Of Chlorine Is Higher Than Sulphur It can also be used to predict if the. examples include most covalent bonds. Because sr lies far to the left of. 3.16 is the electronegativity value of chlorine (cl). The suggested values are all. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. 103 rows electronegativity is. Electronegativity Of Chlorine Is Higher Than Sulphur.
From www.coursehero.com
[Solved] . 1. Chlorine has a higher electronegativity than sulfur. T Electronegativity Of Chlorine Is Higher Than Sulphur Because sr lies far to the left of. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 3.16 is the electronegativity value of chlorine (cl). electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond.. Electronegativity Of Chlorine Is Higher Than Sulphur.
From www.slideshare.net
Electronegativity part two Electronegativity Of Chlorine Is Higher Than Sulphur 3.16 is the electronegativity value of chlorine (cl). The suggested values are all. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. examples include most covalent bonds. It can also be used to predict if the. electronegativity is a chemical property that measures how likely an atom is. Electronegativity Of Chlorine Is Higher Than Sulphur.
From www.youtube.com
Why electronegativity of chlorine is greater than of fluorine Electronegativity Of Chlorine Is Higher Than Sulphur 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. electronegativity is a chemical property that. Electronegativity Of Chlorine Is Higher Than Sulphur.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Electronegativity Of Chlorine Is Higher Than Sulphur 103 rows electronegativity is not a uniquely defined property and may depend on the definition. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond. . Electronegativity Of Chlorine Is Higher Than Sulphur.
From www.dreamstime.com
Chlorine Chemical Element with 17 Atomic Number, Atomic Mass and Electronegativity Of Chlorine Is Higher Than Sulphur 103 rows electronegativity is not a uniquely defined property and may depend on the definition. examples include most covalent bonds. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons. Electronegativity Of Chlorine Is Higher Than Sulphur.
From www.bigstockphoto.com
Electronegativity Image & Photo (Free Trial) Bigstock Electronegativity Of Chlorine Is Higher Than Sulphur 3.16 is the electronegativity value of chlorine (cl). 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). Because sr lies far to the left of. It belongs to the 7th group and 2nd period. Electronegativity Of Chlorine Is Higher Than Sulphur.
From dxoddvwxb.blob.core.windows.net
Magnesium Chloride Electronegativity at Herman blog Electronegativity Of Chlorine Is Higher Than Sulphur electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Because sr lies far to the left of.. Electronegativity Of Chlorine Is Higher Than Sulphur.
From www.numerade.com
SOLVED The bond between sulfur (electronegativity value 2.5) and Electronegativity Of Chlorine Is Higher Than Sulphur It can also be used to predict if the. Because sr lies far to the left of. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The suggested values are all. It belongs to the. Electronegativity Of Chlorine Is Higher Than Sulphur.
From www.meta-synthesis.com
Electronegativity Chemogenesis Electronegativity Of Chlorine Is Higher Than Sulphur The suggested values are all. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It can also be used to predict if the. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. electronegativity is a chemical property that measures how likely an atom. Electronegativity Of Chlorine Is Higher Than Sulphur.
From dxolxdofb.blob.core.windows.net
Electronegativity Chlorine And Bromine at Tammy Parker blog Electronegativity Of Chlorine Is Higher Than Sulphur examples include most covalent bonds. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The suggested values are all. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. electronegativity is a chemical property that measures how likely an atom is. Electronegativity Of Chlorine Is Higher Than Sulphur.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of Electronegativity Of Chlorine Is Higher Than Sulphur Because sr lies far to the left of. electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. electronegativity is a measure of the tendency of an. Electronegativity Of Chlorine Is Higher Than Sulphur.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Electronegativity Of Chlorine Is Higher Than Sulphur It can also be used to predict if the. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Because sr lies far to the left of. electronegativity is a measure of the tendency. Electronegativity Of Chlorine Is Higher Than Sulphur.
From www.dreamstime.com
Chlorine Chemical Element with First Ionization Energy, Atomic Mass and Electronegativity Of Chlorine Is Higher Than Sulphur electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). examples include most covalent bonds. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. It can also be. Electronegativity Of Chlorine Is Higher Than Sulphur.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Electronegativity Of Chlorine Is Higher Than Sulphur electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. electronegativity is a chemical property that measures. Electronegativity Of Chlorine Is Higher Than Sulphur.
From learnwithdrscott.com
Electronegativity Table Easy Hard Science Electronegativity Of Chlorine Is Higher Than Sulphur 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). The suggested values are all. 3.16 is the electronegativity value of chlorine (cl). electronegativity is a measure of the tendency of an atom to. Electronegativity Of Chlorine Is Higher Than Sulphur.
From learningschoolpistadasso.z22.web.core.windows.net
Electronegativity And Bond Polarity Chart Electronegativity Of Chlorine Is Higher Than Sulphur It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond. examples include most covalent bonds. The suggested values are all. electronegativity is a measure of. Electronegativity Of Chlorine Is Higher Than Sulphur.
From www.numerade.com
SOLVED For the compounds HCI and SO2, with the element information Electronegativity Of Chlorine Is Higher Than Sulphur The suggested values are all. 3.16 is the electronegativity value of chlorine (cl). electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond. 119. Electronegativity Of Chlorine Is Higher Than Sulphur.
From chemdictionary.org
Electronegativity Definition And Examples Chemistry Dictionary Electronegativity Of Chlorine Is Higher Than Sulphur 3.16 is the electronegativity value of chlorine (cl). electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Because sr lies far to the left of. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. 103 rows electronegativity is not a. Electronegativity Of Chlorine Is Higher Than Sulphur.
From slideplayer.com
Cover Page Chapter 8 Bonding General Concepts ppt download Electronegativity Of Chlorine Is Higher Than Sulphur electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons.. Electronegativity Of Chlorine Is Higher Than Sulphur.