Solid And Liquid Phases In Equilibrium . distinct liquid phases, two regions of a solid with distinctly different composition or structure separated by boundaries. Equilibrium between two phases means that \(p\), \(t\), and \(\mu(p,t)\) are identical. in figure \(\pageindex{1}\), the line that connects points a and d separates the solid and liquid phases and shows how the melting point of a solid varies with. a key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume,. In particular, crystalline solids having different crystal structure. below the solidus temperature only solid exists; solids are treated in the same way as liquids. It is a familiar fact that pure substances tend to exist in one of three distinct states:
from igcsechemistryrevision.weebly.com
It is a familiar fact that pure substances tend to exist in one of three distinct states: a key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume,. In particular, crystalline solids having different crystal structure. solids are treated in the same way as liquids. Equilibrium between two phases means that \(p\), \(t\), and \(\mu(p,t)\) are identical. below the solidus temperature only solid exists; in figure \(\pageindex{1}\), the line that connects points a and d separates the solid and liquid phases and shows how the melting point of a solid varies with. distinct liquid phases, two regions of a solid with distinctly different composition or structure separated by boundaries.
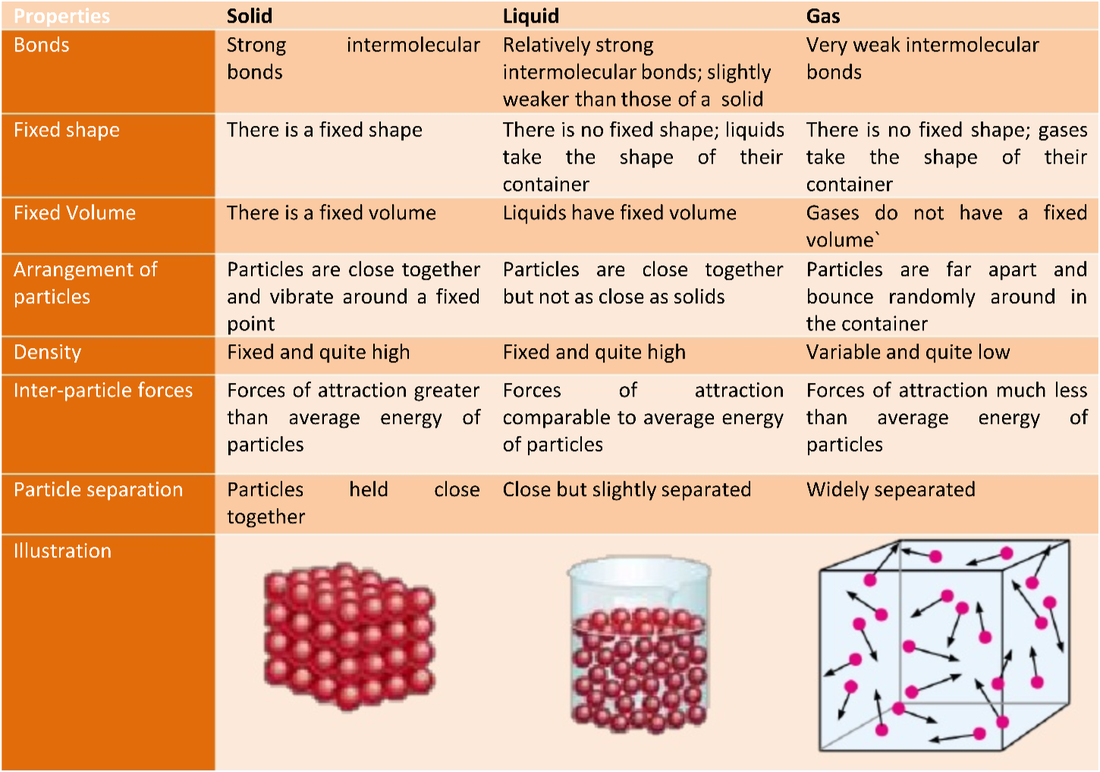
1.1 Understand the arrangement, movement and energy of particles in
Solid And Liquid Phases In Equilibrium In particular, crystalline solids having different crystal structure. below the solidus temperature only solid exists; in figure \(\pageindex{1}\), the line that connects points a and d separates the solid and liquid phases and shows how the melting point of a solid varies with. Equilibrium between two phases means that \(p\), \(t\), and \(\mu(p,t)\) are identical. solids are treated in the same way as liquids. distinct liquid phases, two regions of a solid with distinctly different composition or structure separated by boundaries. In particular, crystalline solids having different crystal structure. It is a familiar fact that pure substances tend to exist in one of three distinct states: a key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume,.
From fphoto.photoshelter.com
science chemistry experiment states of matter Fundamental Photographs Solid And Liquid Phases In Equilibrium In particular, crystalline solids having different crystal structure. distinct liquid phases, two regions of a solid with distinctly different composition or structure separated by boundaries. It is a familiar fact that pure substances tend to exist in one of three distinct states: solids are treated in the same way as liquids. below the solidus temperature only solid. Solid And Liquid Phases In Equilibrium.
From schematiclistsalem123.z22.web.core.windows.net
Phase Diagram Of Chocolate Solid And Liquid Phases In Equilibrium In particular, crystalline solids having different crystal structure. a key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume,. solids are treated in the same way as liquids. distinct liquid phases, two regions of a solid with distinctly different composition or structure separated by boundaries. in figure. Solid And Liquid Phases In Equilibrium.
From javalab.org
Gas, Liquid, Solid Simulation Javalab Solid And Liquid Phases In Equilibrium below the solidus temperature only solid exists; In particular, crystalline solids having different crystal structure. Equilibrium between two phases means that \(p\), \(t\), and \(\mu(p,t)\) are identical. distinct liquid phases, two regions of a solid with distinctly different composition or structure separated by boundaries. It is a familiar fact that pure substances tend to exist in one of. Solid And Liquid Phases In Equilibrium.
From www.youtube.com
SolidLiquid Chemical Equilibrium YouTube Solid And Liquid Phases In Equilibrium solids are treated in the same way as liquids. In particular, crystalline solids having different crystal structure. It is a familiar fact that pure substances tend to exist in one of three distinct states: Equilibrium between two phases means that \(p\), \(t\), and \(\mu(p,t)\) are identical. in figure \(\pageindex{1}\), the line that connects points a and d separates. Solid And Liquid Phases In Equilibrium.
From www.researchgate.net
Experimental solidliquid equilibrium (SLE) phase diagrams ( ) of the Solid And Liquid Phases In Equilibrium a key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume,. below the solidus temperature only solid exists; In particular, crystalline solids having different crystal structure. It is a familiar fact that pure substances tend to exist in one of three distinct states: Equilibrium between two phases means that. Solid And Liquid Phases In Equilibrium.
From kyleniemeyer.github.io
phase equilibrium — Computational Thermodynamics Solid And Liquid Phases In Equilibrium below the solidus temperature only solid exists; distinct liquid phases, two regions of a solid with distinctly different composition or structure separated by boundaries. solids are treated in the same way as liquids. It is a familiar fact that pure substances tend to exist in one of three distinct states: Equilibrium between two phases means that \(p\),. Solid And Liquid Phases In Equilibrium.
From middleschoolscience.com
Solid, Liquid, & Gas Triple Venn Diagram Activity Middle School Solid And Liquid Phases In Equilibrium in figure \(\pageindex{1}\), the line that connects points a and d separates the solid and liquid phases and shows how the melting point of a solid varies with. distinct liquid phases, two regions of a solid with distinctly different composition or structure separated by boundaries. a key tool in exploring phase equilibria is a phase diagram which. Solid And Liquid Phases In Equilibrium.
From www.reddit.com
Can every atom exist in all three states? (Gas,liquid,solid) askscience Solid And Liquid Phases In Equilibrium in figure \(\pageindex{1}\), the line that connects points a and d separates the solid and liquid phases and shows how the melting point of a solid varies with. solids are treated in the same way as liquids. In particular, crystalline solids having different crystal structure. a key tool in exploring phase equilibria is a phase diagram which. Solid And Liquid Phases In Equilibrium.
From understandingsociety.blogspot.com
Understanding Society DeLanda on concepts, knobs, and phase transitions Solid And Liquid Phases In Equilibrium In particular, crystalline solids having different crystal structure. below the solidus temperature only solid exists; solids are treated in the same way as liquids. Equilibrium between two phases means that \(p\), \(t\), and \(\mu(p,t)\) are identical. distinct liquid phases, two regions of a solid with distinctly different composition or structure separated by boundaries. a key tool. Solid And Liquid Phases In Equilibrium.
From manualfixageundeployed.z13.web.core.windows.net
Liquid Liquid Phase Diagram Solid And Liquid Phases In Equilibrium solids are treated in the same way as liquids. In particular, crystalline solids having different crystal structure. Equilibrium between two phases means that \(p\), \(t\), and \(\mu(p,t)\) are identical. a key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume,. in figure \(\pageindex{1}\), the line that connects points. Solid And Liquid Phases In Equilibrium.
From schematichettum57.z21.web.core.windows.net
How To Read Phase Diagrams Chemistry Solid And Liquid Phases In Equilibrium solids are treated in the same way as liquids. distinct liquid phases, two regions of a solid with distinctly different composition or structure separated by boundaries. It is a familiar fact that pure substances tend to exist in one of three distinct states: a key tool in exploring phase equilibria is a phase diagram which is used. Solid And Liquid Phases In Equilibrium.
From www.slideserve.com
PPT Phase Equilibrium PowerPoint Presentation, free download ID2503286 Solid And Liquid Phases In Equilibrium below the solidus temperature only solid exists; It is a familiar fact that pure substances tend to exist in one of three distinct states: Equilibrium between two phases means that \(p\), \(t\), and \(\mu(p,t)\) are identical. a key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume,. In particular,. Solid And Liquid Phases In Equilibrium.
From unistudium.unipg.it
Phase Diagrams Solid And Liquid Phases In Equilibrium below the solidus temperature only solid exists; a key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume,. In particular, crystalline solids having different crystal structure. solids are treated in the same way as liquids. It is a familiar fact that pure substances tend to exist in one. Solid And Liquid Phases In Equilibrium.
From www.researchgate.net
Phase diagram and the phase equilibrium line 1 solid and liquid Solid And Liquid Phases In Equilibrium It is a familiar fact that pure substances tend to exist in one of three distinct states: solids are treated in the same way as liquids. In particular, crystalline solids having different crystal structure. below the solidus temperature only solid exists; distinct liquid phases, two regions of a solid with distinctly different composition or structure separated by. Solid And Liquid Phases In Equilibrium.
From shaunmwilliams.com
Chapter 1 Presentation Solid And Liquid Phases In Equilibrium solids are treated in the same way as liquids. Equilibrium between two phases means that \(p\), \(t\), and \(\mu(p,t)\) are identical. below the solidus temperature only solid exists; a key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume,. distinct liquid phases, two regions of a solid. Solid And Liquid Phases In Equilibrium.
From physicalpharmacy1424.blogspot.com
Physical Pharmacy Practical Practical 2 Phase Diagram Mutual Solid And Liquid Phases In Equilibrium a key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume,. In particular, crystalline solids having different crystal structure. distinct liquid phases, two regions of a solid with distinctly different composition or structure separated by boundaries. It is a familiar fact that pure substances tend to exist in one. Solid And Liquid Phases In Equilibrium.
From eleccircs.com
An exploration of binary liquid phase diagrams Solid And Liquid Phases In Equilibrium a key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume,. In particular, crystalline solids having different crystal structure. It is a familiar fact that pure substances tend to exist in one of three distinct states: in figure \(\pageindex{1}\), the line that connects points a and d separates the. Solid And Liquid Phases In Equilibrium.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Solid And Liquid Phases In Equilibrium in figure \(\pageindex{1}\), the line that connects points a and d separates the solid and liquid phases and shows how the melting point of a solid varies with. It is a familiar fact that pure substances tend to exist in one of three distinct states: below the solidus temperature only solid exists; solids are treated in the. Solid And Liquid Phases In Equilibrium.
From fixlibrarycoblasbi.z13.web.core.windows.net
How To Read Phase Diagrams Chemistry Solid And Liquid Phases In Equilibrium distinct liquid phases, two regions of a solid with distinctly different composition or structure separated by boundaries. solids are treated in the same way as liquids. in figure \(\pageindex{1}\), the line that connects points a and d separates the solid and liquid phases and shows how the melting point of a solid varies with. Equilibrium between two. Solid And Liquid Phases In Equilibrium.
From schematicviciosinfin17.z22.web.core.windows.net
How To Make A Phase Diagram Solid And Liquid Phases In Equilibrium distinct liquid phases, two regions of a solid with distinctly different composition or structure separated by boundaries. below the solidus temperature only solid exists; in figure \(\pageindex{1}\), the line that connects points a and d separates the solid and liquid phases and shows how the melting point of a solid varies with. It is a familiar fact. Solid And Liquid Phases In Equilibrium.
From studylib.net
Equilibrium for a general reaction Solid And Liquid Phases In Equilibrium In particular, crystalline solids having different crystal structure. a key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume,. Equilibrium between two phases means that \(p\), \(t\), and \(\mu(p,t)\) are identical. below the solidus temperature only solid exists; in figure \(\pageindex{1}\), the line that connects points a and. Solid And Liquid Phases In Equilibrium.
From chemistry.stackexchange.com
liquids How can CO2 exist in two different phases? Chemistry Stack Solid And Liquid Phases In Equilibrium below the solidus temperature only solid exists; It is a familiar fact that pure substances tend to exist in one of three distinct states: distinct liquid phases, two regions of a solid with distinctly different composition or structure separated by boundaries. a key tool in exploring phase equilibria is a phase diagram which is used to show. Solid And Liquid Phases In Equilibrium.
From www.youtube.com
Intermolecular Forces of Attraction Solids, Liquids, Phases, States Solid And Liquid Phases In Equilibrium It is a familiar fact that pure substances tend to exist in one of three distinct states: solids are treated in the same way as liquids. distinct liquid phases, two regions of a solid with distinctly different composition or structure separated by boundaries. below the solidus temperature only solid exists; a key tool in exploring phase. Solid And Liquid Phases In Equilibrium.
From learncheme.com
ssleequilibrium LearnChemE Solid And Liquid Phases In Equilibrium distinct liquid phases, two regions of a solid with distinctly different composition or structure separated by boundaries. In particular, crystalline solids having different crystal structure. It is a familiar fact that pure substances tend to exist in one of three distinct states: a key tool in exploring phase equilibria is a phase diagram which is used to show. Solid And Liquid Phases In Equilibrium.
From igcsechemistryrevision.weebly.com
1.1 Understand the arrangement, movement and energy of particles in Solid And Liquid Phases In Equilibrium distinct liquid phases, two regions of a solid with distinctly different composition or structure separated by boundaries. It is a familiar fact that pure substances tend to exist in one of three distinct states: in figure \(\pageindex{1}\), the line that connects points a and d separates the solid and liquid phases and shows how the melting point of. Solid And Liquid Phases In Equilibrium.
From chem.libretexts.org
Chapter 7.7 Phase Diagrams Chemistry LibreTexts Solid And Liquid Phases In Equilibrium solids are treated in the same way as liquids. distinct liquid phases, two regions of a solid with distinctly different composition or structure separated by boundaries. a key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume,. in figure \(\pageindex{1}\), the line that connects points a and. Solid And Liquid Phases In Equilibrium.
From learncheme.com
solidsolidliquidphasediagramsconceptestandexampleproblem Solid And Liquid Phases In Equilibrium Equilibrium between two phases means that \(p\), \(t\), and \(\mu(p,t)\) are identical. In particular, crystalline solids having different crystal structure. below the solidus temperature only solid exists; It is a familiar fact that pure substances tend to exist in one of three distinct states: a key tool in exploring phase equilibria is a phase diagram which is used. Solid And Liquid Phases In Equilibrium.
From www.youtube.com
Partitioning Between Liquid Phases YouTube Solid And Liquid Phases In Equilibrium In particular, crystalline solids having different crystal structure. solids are treated in the same way as liquids. below the solidus temperature only solid exists; It is a familiar fact that pure substances tend to exist in one of three distinct states: in figure \(\pageindex{1}\), the line that connects points a and d separates the solid and liquid. Solid And Liquid Phases In Equilibrium.
From www.youtube.com
Solidliquid phase diagrams YouTube Solid And Liquid Phases In Equilibrium In particular, crystalline solids having different crystal structure. Equilibrium between two phases means that \(p\), \(t\), and \(\mu(p,t)\) are identical. a key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume,. solids are treated in the same way as liquids. in figure \(\pageindex{1}\), the line that connects points. Solid And Liquid Phases In Equilibrium.
From www.mdpi.com
Molecules Free FullText Modeling of SolidLiquid Equilibria in Solid And Liquid Phases In Equilibrium In particular, crystalline solids having different crystal structure. distinct liquid phases, two regions of a solid with distinctly different composition or structure separated by boundaries. below the solidus temperature only solid exists; Equilibrium between two phases means that \(p\), \(t\), and \(\mu(p,t)\) are identical. a key tool in exploring phase equilibria is a phase diagram which is. Solid And Liquid Phases In Equilibrium.
From www.researchgate.net
Equilibrium concentrations of the solid and the liquid phase for the Solid And Liquid Phases In Equilibrium solids are treated in the same way as liquids. in figure \(\pageindex{1}\), the line that connects points a and d separates the solid and liquid phases and shows how the melting point of a solid varies with. Equilibrium between two phases means that \(p\), \(t\), and \(\mu(p,t)\) are identical. In particular, crystalline solids having different crystal structure. . Solid And Liquid Phases In Equilibrium.
From demonstrations.wolfram.com
LiquidLiquid Equilibrium Diagrams for Ternary Mixtures Wolfram Solid And Liquid Phases In Equilibrium distinct liquid phases, two regions of a solid with distinctly different composition or structure separated by boundaries. In particular, crystalline solids having different crystal structure. in figure \(\pageindex{1}\), the line that connects points a and d separates the solid and liquid phases and shows how the melting point of a solid varies with. Equilibrium between two phases means. Solid And Liquid Phases In Equilibrium.
From www.slideserve.com
PPT Equilibrium Conversion PowerPoint Presentation, free download Solid And Liquid Phases In Equilibrium distinct liquid phases, two regions of a solid with distinctly different composition or structure separated by boundaries. In particular, crystalline solids having different crystal structure. It is a familiar fact that pure substances tend to exist in one of three distinct states: in figure \(\pageindex{1}\), the line that connects points a and d separates the solid and liquid. Solid And Liquid Phases In Equilibrium.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Solid And Liquid Phases In Equilibrium in figure \(\pageindex{1}\), the line that connects points a and d separates the solid and liquid phases and shows how the melting point of a solid varies with. In particular, crystalline solids having different crystal structure. below the solidus temperature only solid exists; distinct liquid phases, two regions of a solid with distinctly different composition or structure. Solid And Liquid Phases In Equilibrium.
From schematicdatabitos99.z22.web.core.windows.net
Liquid Liquid Phase Diagram Solid And Liquid Phases In Equilibrium In particular, crystalline solids having different crystal structure. in figure \(\pageindex{1}\), the line that connects points a and d separates the solid and liquid phases and shows how the melting point of a solid varies with. a key tool in exploring phase equilibria is a phase diagram which is used to show conditions (pressure, temperature, volume,. It is. Solid And Liquid Phases In Equilibrium.