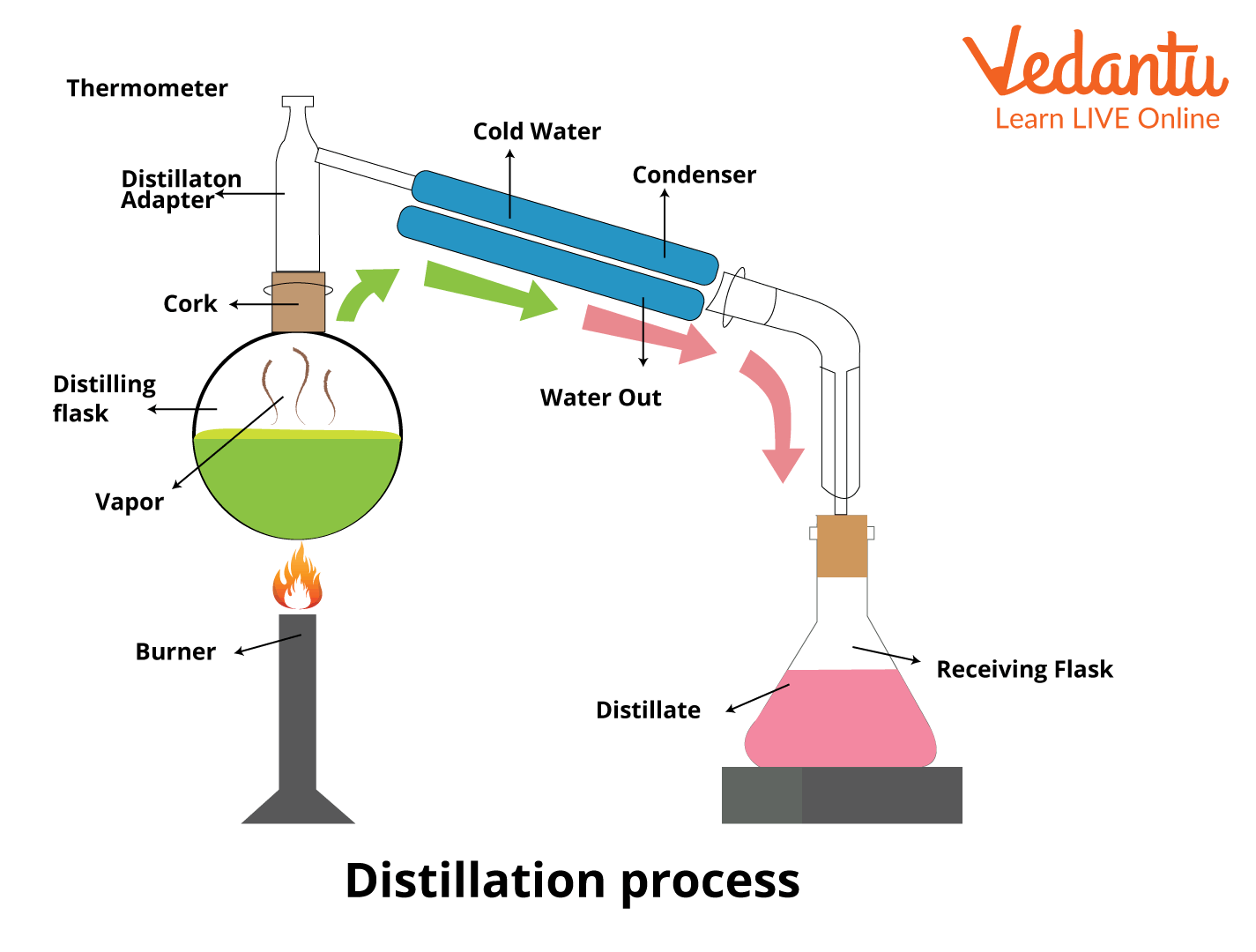
The Definition Of Vacuum Distillation . a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound (or the. Overview of vacuum distillation boiling commences when the vapor pressure of a liquid or solution equals the external or. A fraction distillation can also be used. vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. In other words, distillation under reduced pressure is known as vacuum distillation. a vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). This is performed by lowering the pressures in the column or the reactor. the principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation. vacuum distillation definition. When the pressure is lowered inside the apparatus, solutions boil at a lower temperature.
from www.vrogue.co
a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound (or the. Overview of vacuum distillation boiling commences when the vapor pressure of a liquid or solution equals the external or. a vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). A fraction distillation can also be used. vacuum distillation definition. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. In other words, distillation under reduced pressure is known as vacuum distillation. the principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation. This is performed by lowering the pressures in the column or the reactor.
Types Of Distillation Definition Process Uses Example vrogue.co
The Definition Of Vacuum Distillation This is performed by lowering the pressures in the column or the reactor. A fraction distillation can also be used. the principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Overview of vacuum distillation boiling commences when the vapor pressure of a liquid or solution equals the external or. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound (or the. This is performed by lowering the pressures in the column or the reactor. a vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). vacuum distillation definition. In other words, distillation under reduced pressure is known as vacuum distillation. When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation.
From www.simplepharmanotes.com
Distillation under reduced pressure / Vacuum Distillation. The Definition Of Vacuum Distillation A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. a vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). Overview of vacuum distillation boiling commences when the vapor pressure of a liquid or solution equals the external or. When the pressure. The Definition Of Vacuum Distillation.
From www.pinterest.com
The Various Types of Distillation That are Worth Knowing Distillation The Definition Of Vacuum Distillation a vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above. The Definition Of Vacuum Distillation.
From www.researchgate.net
Vacuum distillation apparatus. Download Scientific Diagram The Definition Of Vacuum Distillation vacuum distillation definition. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower. The Definition Of Vacuum Distillation.
From elitetradebd.com
Vacuum Distillation Glass Elite Scientific & Meditech Co. The Definition Of Vacuum Distillation vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. This is performed by lowering the pressures in the column or the reactor. Overview of vacuum distillation. The Definition Of Vacuum Distillation.
From easywayscience78.blogspot.com
Distillation Easy way to learn science The Definition Of Vacuum Distillation vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling. a vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). This is performed by lowering the pressures in the. The Definition Of Vacuum Distillation.
From foodtechnotes.com
Distillation Principle and Types Food Tech Notes The Definition Of Vacuum Distillation A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. the principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation. a vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). Overview of vacuum distillation boiling commences when. The Definition Of Vacuum Distillation.
From www.researchgate.net
Diagram of the vacuum distillation still used for purification The Definition Of Vacuum Distillation Overview of vacuum distillation boiling commences when the vapor pressure of a liquid or solution equals the external or. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. A fraction distillation can also be used. In other words, distillation under reduced pressure is known as vacuum distillation. When the pressure is lowered inside. The Definition Of Vacuum Distillation.
From www.vrogue.co
Types Of Distillation Definition Process Uses Example vrogue.co The Definition Of Vacuum Distillation a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound (or the. A fraction distillation can also be used. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Overview of vacuum distillation boiling commences when. The Definition Of Vacuum Distillation.
From www.thoughtco.com
What Is Distillation? Principles and Uses The Definition Of Vacuum Distillation a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound (or the. A fraction distillation can also be used. This is performed by lowering the pressures in the column or the reactor. Overview of vacuum distillation boiling commences when the. The Definition Of Vacuum Distillation.
From www.youtube.com
What is vacuum distillation use of vacuum distillation what is air The Definition Of Vacuum Distillation Overview of vacuum distillation boiling commences when the vapor pressure of a liquid or solution equals the external or. vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling. This is performed by lowering the pressures in the column or the reactor. . The Definition Of Vacuum Distillation.
From chemicaltweak.com
When Vacuum Distillation Is Selected Application And Uses Of VDU The Definition Of Vacuum Distillation A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound (or the. This is performed by lowering the pressures in the column or the reactor. When. The Definition Of Vacuum Distillation.
From chemicaltweak.com
Vacuum Distillation Process And Working Principle VDU Full Form The Definition Of Vacuum Distillation A fraction distillation can also be used. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. the principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation. vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with. The Definition Of Vacuum Distillation.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry The Definition Of Vacuum Distillation This is performed by lowering the pressures in the column or the reactor. Overview of vacuum distillation boiling commences when the vapor pressure of a liquid or solution equals the external or. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. A fraction distillation can also be used. In other words, distillation under. The Definition Of Vacuum Distillation.
From www.alamy.com
Vacuum Distillation System Stock Vector Image & Art Alamy The Definition Of Vacuum Distillation a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound (or the. In other words, distillation under reduced pressure is known as vacuum distillation. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation.. The Definition Of Vacuum Distillation.
From www.geeksforgeeks.org
Fractional Distillation Definition, Principle, & Applications The Definition Of Vacuum Distillation the principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation. Overview of vacuum distillation boiling commences when the vapor pressure of a liquid or solution equals the external or. In other words, distillation under reduced pressure is known as vacuum distillation. a vacuum distillation is used when the boiling point of the compound (or. The Definition Of Vacuum Distillation.
From blog.digivac.com
3 Benefits of Vacuum Distillation in Low and Medium Vacuum The Definition Of Vacuum Distillation a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound (or the. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. A fraction distillation can also be used. Overview of vacuum distillation boiling commences when. The Definition Of Vacuum Distillation.
From glossary.periodni.com
Chemistry Glossary Search results for 'distillation' The Definition Of Vacuum Distillation a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound (or the. In other words, distillation under reduced pressure is known as vacuum distillation. vacuum distillation definition. a vacuum distillation is performed by applying a vacuum source to. The Definition Of Vacuum Distillation.
From www.geeksforgeeks.org
Methods of Purification of Organic Compounds The Definition Of Vacuum Distillation When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. In other words, distillation under reduced pressure is known as vacuum distillation. a vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or. The Definition Of Vacuum Distillation.
From www.researchgate.net
The schematic for the vacuum distillation process. Download The Definition Of Vacuum Distillation A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. a vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). In other words, distillation under reduced pressure is known as vacuum distillation. vacuum distillation is a technique for the purification or. The Definition Of Vacuum Distillation.
From naizaklab.com
High Vacuum Distillation units Naizak Lab The Definition Of Vacuum Distillation In other words, distillation under reduced pressure is known as vacuum distillation. A fraction distillation can also be used. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to. The Definition Of Vacuum Distillation.
From safrole.com
Vacuum distillation Safrole The Definition Of Vacuum Distillation This is performed by lowering the pressures in the column or the reactor. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling. Overview of vacuum distillation boiling commences. The Definition Of Vacuum Distillation.
From thepetrosolutions.com
Vacuum Distillation Unit in Oil Refinery The Petro Solutions The Definition Of Vacuum Distillation the principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation. This is performed by lowering the pressures in the column or the reactor. When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. Overview of vacuum distillation boiling commences when the vapor pressure of a liquid or solution equals the external. The Definition Of Vacuum Distillation.
From www.youtube.com
Vacuum Distillation YouTube The Definition Of Vacuum Distillation When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound (or the. This is performed by lowering the pressures in the column or the reactor. In. The Definition Of Vacuum Distillation.
From chemicaltweak.com
6 Types Of Distillation And Definition [Explained In Detail] The Definition Of Vacuum Distillation This is performed by lowering the pressures in the column or the reactor. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. Overview of vacuum distillation boiling commences when the vapor pressure of a liquid or solution equals the external or. vacuum distillation is a technique for the purification or separation of. The Definition Of Vacuum Distillation.
From www.shutterstock.com
Distillation Distillation Under Reduced Pressure Vacuum Stock Vector The Definition Of Vacuum Distillation a vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). In other words, distillation under reduced pressure is known as vacuum distillation. When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. a vacuum distillation is used when the boiling point. The Definition Of Vacuum Distillation.
From www.pressurecontrolsolutions.com
Vacuum Distillation issues? Call Pressure Control Solutions! The Definition Of Vacuum Distillation vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling. This is performed by lowering the pressures in the column or the reactor. A fraction distillation can also be used. A distillation operation that is performed at a pressure below atmospheric pressure is. The Definition Of Vacuum Distillation.
From chemicaltweak.com
6 Types Of Distillation And Definition [Explained In Detail] The Definition Of Vacuum Distillation A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. the principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation. When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. a vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either. The Definition Of Vacuum Distillation.
From supertekglassware.com
Vacuum Distillation The Definition Of Vacuum Distillation the principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. A fraction distillation can also be used. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150. The Definition Of Vacuum Distillation.
From www.chemicals.co.uk
Distillation Of A Product From A Reaction The Chemistry Blog The Definition Of Vacuum Distillation Overview of vacuum distillation boiling commences when the vapor pressure of a liquid or solution equals the external or. When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling.. The Definition Of Vacuum Distillation.
From slidetodoc.com
DISTILLATION DEFINITION Distillation is an unit operation which The Definition Of Vacuum Distillation the principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation. vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling. This is performed by lowering the pressures in the column or the reactor. When the pressure is lowered. The Definition Of Vacuum Distillation.
From www.researchgate.net
The vacuum distillation apparatus scheme. Download Scientific Diagram The Definition Of Vacuum Distillation A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. the principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the. The Definition Of Vacuum Distillation.
From www.e-education.psu.edu
Atmospheric and Vacuum Distillation Units FSC 432 Petroleum Refining The Definition Of Vacuum Distillation When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. In other words, distillation under reduced pressure is known as vacuum distillation. vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling. A vacuum distillation apparatus is shown in. The Definition Of Vacuum Distillation.
From pediaa.com
Difference Between Steam Distillation and Fractional Distillation The Definition Of Vacuum Distillation a vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150. The Definition Of Vacuum Distillation.
From www.researchgate.net
Process flow diagram of Vacuum Distillation Unit Download Scientific The Definition Of Vacuum Distillation a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t b >150 o c) in order to distill the compound (or the. When the pressure is lowered inside the apparatus, solutions boil at a lower temperature. In other words, distillation under reduced pressure is known as vacuum distillation. This is. The Definition Of Vacuum Distillation.
From eureka.patsnap.com
Atmospheric vacuum distillation process for light crude oil Eureka The Definition Of Vacuum Distillation the principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation. This is performed by lowering the pressures in the column or the reactor. Overview of vacuum distillation boiling commences when the vapor pressure of a liquid or solution equals the external or. a vacuum distillation is performed by applying a vacuum source to the. The Definition Of Vacuum Distillation.