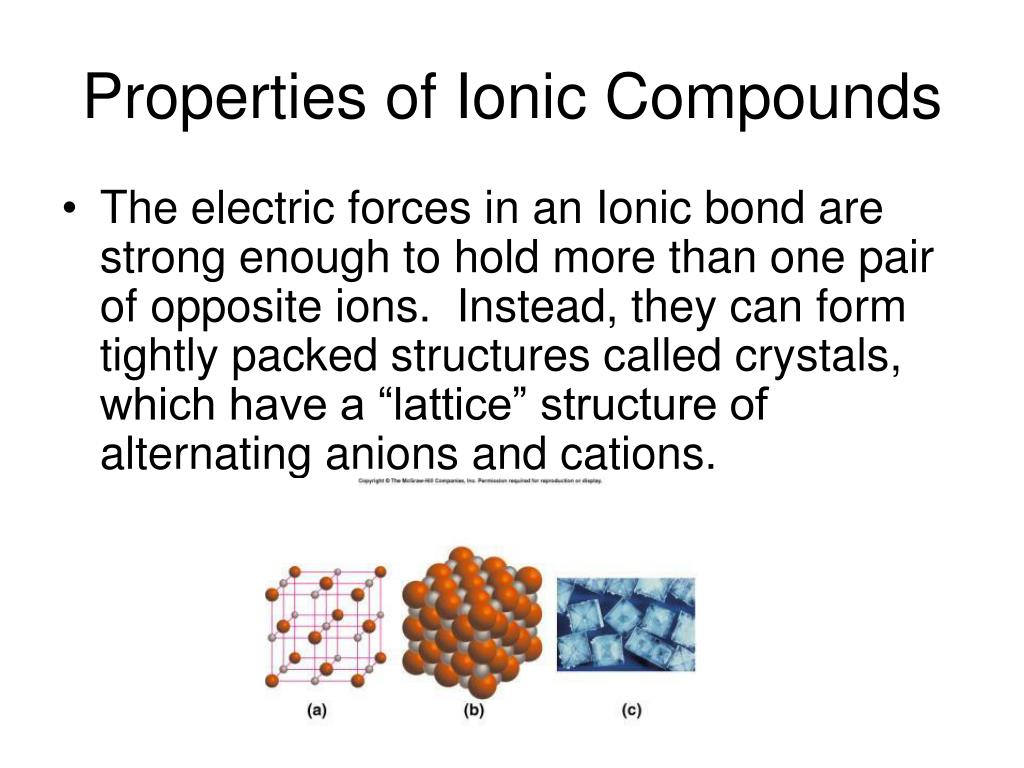
Why Are Ionic Compounds Brittle And Metals Malleable . Explain why ionic substances are brittle, and metals are malleable. Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds are not unusual in being brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of. Gold, silver, aluminum, iron, and copper are malleable. Malleable capable of being hammered or pressed into a new shape without being likely to break or return to the original shape. A metal behaves as an array of metal. Recall that ionic compounds are very brittle. Ionic compounds are generally hard, but brittle. Your starting assumption that ionic compounds are frequently brittle is misleading.
from www.slideserve.com
It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of. Explain why ionic substances are brittle, and metals are malleable. Ionic compounds are generally hard, but brittle. Ionic compounds are not unusual in being brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one. Your starting assumption that ionic compounds are frequently brittle is misleading. Ionic compounds are generally hard, but brittle. Recall that ionic compounds are very brittle. A metal behaves as an array of metal. Gold, silver, aluminum, iron, and copper are malleable.
PPT Ionic Compounds Notes PowerPoint Presentation, free download ID
Why Are Ionic Compounds Brittle And Metals Malleable Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Malleable capable of being hammered or pressed into a new shape without being likely to break or return to the original shape. Explain why ionic substances are brittle, and metals are malleable. A metal behaves as an array of metal. Ionic compounds are not unusual in being brittle. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of. Your starting assumption that ionic compounds are frequently brittle is misleading. Ionic compounds are generally hard, but brittle. Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one. Recall that ionic compounds are very brittle. Gold, silver, aluminum, iron, and copper are malleable.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5980343 Why Are Ionic Compounds Brittle And Metals Malleable Ionic compounds are generally hard, but brittle. Ionic compounds are not unusual in being brittle. A metal behaves as an array of metal. Gold, silver, aluminum, iron, and copper are malleable. Ionic compounds are generally hard, but brittle. Explain why ionic substances are brittle, and metals are malleable. Ionic compounds are brittle due to the strong bond between the positive. Why Are Ionic Compounds Brittle And Metals Malleable.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Why Are Ionic Compounds Brittle And Metals Malleable Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of. Ionic compounds are generally hard, but brittle. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Your starting assumption that ionic. Why Are Ionic Compounds Brittle And Metals Malleable.
From slideplayer.com
Ionic Bonding/Naming. ppt download Why Are Ionic Compounds Brittle And Metals Malleable Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one.. Why Are Ionic Compounds Brittle And Metals Malleable.
From slideplayer.com
Metallic Bonding. ppt download Why Are Ionic Compounds Brittle And Metals Malleable It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules.. Why Are Ionic Compounds Brittle And Metals Malleable.
From www.slideserve.com
PPT Properties of ionic compounds PowerPoint Presentation ID1130121 Why Are Ionic Compounds Brittle And Metals Malleable A metal behaves as an array of metal. Explain why ionic substances are brittle, and metals are malleable. Malleable capable of being hammered or pressed into a new shape without being likely to break or return to the original shape. Recall that ionic compounds are very brittle. It takes a large amount of mechanical force, such as striking a crystal. Why Are Ionic Compounds Brittle And Metals Malleable.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Why Are Ionic Compounds Brittle And Metals Malleable Malleable capable of being hammered or pressed into a new shape without being likely to break or return to the original shape. Explain why ionic substances are brittle, and metals are malleable. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds are generally hard, but brittle. Ionic. Why Are Ionic Compounds Brittle And Metals Malleable.
From slideplayer.com
Ionic bonding. ppt download Why Are Ionic Compounds Brittle And Metals Malleable It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one. Explain why ionic substances are brittle, and metals are malleable. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of. Ionic compounds are not unusual in being brittle. Ionic compounds. Why Are Ionic Compounds Brittle And Metals Malleable.
From www.slideserve.com
PPT Chapter 9 Chemical Bonding I Lewis Theory PowerPoint Why Are Ionic Compounds Brittle And Metals Malleable Ionic compounds are generally hard, but brittle. A metal behaves as an array of metal. Malleable capable of being hammered or pressed into a new shape without being likely to break or return to the original shape. Your starting assumption that ionic compounds are frequently brittle is misleading. It takes a large amount of mechanical force, such as striking a. Why Are Ionic Compounds Brittle And Metals Malleable.
From www.slideserve.com
PPT Properties of Ionic, Covalent, and Metallic Compounds PowerPoint Why Are Ionic Compounds Brittle And Metals Malleable Your starting assumption that ionic compounds are frequently brittle is misleading. Ionic compounds are generally hard, but brittle. Ionic compounds are not unusual in being brittle. Recall that ionic compounds are very brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of. Ionic compounds are generally hard, but. Why Are Ionic Compounds Brittle And Metals Malleable.
From ibstudy.net
4.1 Ionic bonding and structure Wang's website Why Are Ionic Compounds Brittle And Metals Malleable Gold, silver, aluminum, iron, and copper are malleable. Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one. Your starting assumption that ionic compounds are frequently brittle is misleading. Ionic compounds are generally hard, but brittle. Ionic compounds are brittle due to the strong. Why Are Ionic Compounds Brittle And Metals Malleable.
From www.youtube.com
Why ionic solids are brittle? YouTube Why Are Ionic Compounds Brittle And Metals Malleable Recall that ionic compounds are very brittle. Ionic compounds are not unusual in being brittle. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one. Ionic compounds are generally hard, but. Why Are Ionic Compounds Brittle And Metals Malleable.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica Why Are Ionic Compounds Brittle And Metals Malleable Gold, silver, aluminum, iron, and copper are malleable. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one. Explain why ionic substances are brittle, and metals are malleable. Malleable capable. Why Are Ionic Compounds Brittle And Metals Malleable.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica Why Are Ionic Compounds Brittle And Metals Malleable Explain why ionic substances are brittle, and metals are malleable. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of. Malleable capable of being hammered or pressed into a new shape without being likely to break or return to the original shape. Ionic compounds are generally hard, but brittle.. Why Are Ionic Compounds Brittle And Metals Malleable.
From slideplayer.com
Ionic Compounds Chapter ppt download Why Are Ionic Compounds Brittle And Metals Malleable Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one. Malleable capable of being hammered or pressed into a new shape without being likely to break or return to the original shape. Ionic compounds are generally hard, but brittle. Explain why ionic substances are. Why Are Ionic Compounds Brittle And Metals Malleable.
From slideplayer.com
Ionic Bonds. ppt download Why Are Ionic Compounds Brittle And Metals Malleable It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one. Ionic compounds are generally hard, but brittle. A metal behaves as an array of metal. Recall that ionic compounds are very brittle. Your starting assumption that ionic compounds are frequently brittle is misleading. Ionic compounds are not unusual in being brittle.. Why Are Ionic Compounds Brittle And Metals Malleable.
From slidetodoc.com
TYPES OF CHEMICAL BONDS IONIC BONDS COVALENT BONDS Why Are Ionic Compounds Brittle And Metals Malleable It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of. Malleable capable of being hammered or pressed into a new shape without being likely to break or return to the original shape. Explain why ionic substances are brittle, and metals are malleable. Ionic compounds are generally hard, but brittle.. Why Are Ionic Compounds Brittle And Metals Malleable.
From www.shalom-education.com
Properties of Ionic Compounds GCSE Chemistry Revision Why Are Ionic Compounds Brittle And Metals Malleable It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of. Your starting assumption that ionic compounds are frequently brittle is misleading. Malleable capable of being hammered or pressed into a new shape without being likely to break or return to the original shape. Ionic compounds are not unusual in. Why Are Ionic Compounds Brittle And Metals Malleable.
From slideplayer.com
Notes Properties of Ionic Compounds 3 ppt download Why Are Ionic Compounds Brittle And Metals Malleable Ionic compounds are generally hard, but brittle. Ionic compounds are not unusual in being brittle. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one. Gold, silver, aluminum, iron, and copper. Why Are Ionic Compounds Brittle And Metals Malleable.
From www.youtube.com
Examples of Ionic Bonding YouTube Why Are Ionic Compounds Brittle And Metals Malleable Your starting assumption that ionic compounds are frequently brittle is misleading. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of. Explain why ionic substances are brittle, and metals are malleable. Ionic compounds are generally hard, but brittle. Ionic compounds are not unusual in being brittle. Gold, silver, aluminum,. Why Are Ionic Compounds Brittle And Metals Malleable.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? Why Are Ionic Compounds Brittle And Metals Malleable Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds are generally hard, but brittle. Malleable capable of being hammered or pressed into a new shape without being likely to break or return to the original shape. Ionic compounds are not unusual in being brittle. Your starting assumption. Why Are Ionic Compounds Brittle And Metals Malleable.
From slideplayer.com
Metallic Bonds and Properties of Metals ppt video online download Why Are Ionic Compounds Brittle And Metals Malleable Ionic compounds are not unusual in being brittle. Explain why ionic substances are brittle, and metals are malleable. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of. Ionic compounds are generally hard, but brittle. Ionic compounds are brittle due to the strong bond between the positive and negative. Why Are Ionic Compounds Brittle And Metals Malleable.
From www.slideserve.com
PPT Worksheet Chapter 12 Homework Key PowerPoint Presentation, free Why Are Ionic Compounds Brittle And Metals Malleable Malleable capable of being hammered or pressed into a new shape without being likely to break or return to the original shape. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of. Explain why ionic substances are brittle, and metals are malleable. Ionic compounds are generally hard, but brittle.. Why Are Ionic Compounds Brittle And Metals Malleable.
From www.doubtnut.com
Explain why ionic compounds are hard and brittle? Why Are Ionic Compounds Brittle And Metals Malleable Explain why ionic substances are brittle, and metals are malleable. Your starting assumption that ionic compounds are frequently brittle is misleading. A metal behaves as an array of metal. Ionic compounds are not unusual in being brittle. Gold, silver, aluminum, iron, and copper are malleable. Recall that ionic compounds are very brittle. Malleable capable of being hammered or pressed into. Why Are Ionic Compounds Brittle And Metals Malleable.
From www.slideserve.com
PPT Ionic Compounds PowerPoint Presentation, free download ID1996379 Why Are Ionic Compounds Brittle And Metals Malleable A metal behaves as an array of metal. Your starting assumption that ionic compounds are frequently brittle is misleading. Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of. Explain why ionic substances are brittle, and metals are malleable. Gold, silver, aluminum,. Why Are Ionic Compounds Brittle And Metals Malleable.
From slideplayer.com
Chemistry Ionic Compounds. ppt download Why Are Ionic Compounds Brittle And Metals Malleable Recall that ionic compounds are very brittle. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds are generally hard, but brittle. A metal behaves as an array of metal. Gold, silver, aluminum, iron, and copper are malleable. Ionic compounds are not unusual in being brittle. Malleable capable. Why Are Ionic Compounds Brittle And Metals Malleable.
From www.slideserve.com
PPT Ionic Compounds Notes PowerPoint Presentation, free download ID Why Are Ionic Compounds Brittle And Metals Malleable It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one. Your starting assumption that ionic compounds are frequently brittle is misleading. A metal behaves as an array of metal. Recall that ionic compounds are very brittle. Gold, silver, aluminum, iron, and copper are malleable. It takes a large amount of mechanical. Why Are Ionic Compounds Brittle And Metals Malleable.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Why Are Ionic Compounds Brittle And Metals Malleable Malleable capable of being hammered or pressed into a new shape without being likely to break or return to the original shape. Explain why ionic substances are brittle, and metals are malleable. Ionic compounds are generally hard, but brittle. Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with. Why Are Ionic Compounds Brittle And Metals Malleable.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Why Are Ionic Compounds Brittle And Metals Malleable Malleable capable of being hammered or pressed into a new shape without being likely to break or return to the original shape. Recall that ionic compounds are very brittle. Ionic compounds are generally hard, but brittle. Explain why ionic substances are brittle, and metals are malleable. Ionic compounds are brittle due to the strong bond between the positive and negative. Why Are Ionic Compounds Brittle And Metals Malleable.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Why Are Ionic Compounds Brittle And Metals Malleable Ionic compounds are not unusual in being brittle. Gold, silver, aluminum, iron, and copper are malleable. Ionic compounds are generally hard, but brittle. Your starting assumption that ionic compounds are frequently brittle is misleading. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Malleable capable of being hammered or. Why Are Ionic Compounds Brittle And Metals Malleable.
From shareeducatonideas.com
What Is An Ionic Compound? Formula and Defination Why Are Ionic Compounds Brittle And Metals Malleable Gold, silver, aluminum, iron, and copper are malleable. Your starting assumption that ionic compounds are frequently brittle is misleading. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one. Recall that ionic compounds are very brittle. Ionic compounds are not unusual in being brittle. Ionic compounds are generally hard, but brittle.. Why Are Ionic Compounds Brittle And Metals Malleable.
From slideplayer.com
Ionic Bonds Chapter 5 Section ppt download Why Are Ionic Compounds Brittle And Metals Malleable Your starting assumption that ionic compounds are frequently brittle is misleading. Ionic compounds are generally hard, but brittle. Explain why ionic substances are brittle, and metals are malleable. Recall that ionic compounds are very brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of. Ionic compounds are brittle. Why Are Ionic Compounds Brittle And Metals Malleable.
From www.youtube.com
Ionic Bonding Properties of Ionic Compounds YouTube Why Are Ionic Compounds Brittle And Metals Malleable A metal behaves as an array of metal. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of. Malleable capable of being hammered or pressed into a new shape without being likely to break or return to the original shape. Explain why ionic substances are brittle, and metals are. Why Are Ionic Compounds Brittle And Metals Malleable.
From www.slideserve.com
PPT Worksheet Chapter 12 Homework Key PowerPoint Presentation, free Why Are Ionic Compounds Brittle And Metals Malleable It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of. Recall that ionic compounds are very brittle. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds are generally hard, but brittle. A metal behaves as an. Why Are Ionic Compounds Brittle And Metals Malleable.
From slideplayer.com
IONIC COMPOUNDS. ppt download Why Are Ionic Compounds Brittle And Metals Malleable Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds are generally hard, but brittle. Ionic compounds are generally hard, but brittle. Gold, silver, aluminum, iron, and copper are malleable. Recall that ionic compounds are very brittle. It takes a large amount of mechanical force, such as striking. Why Are Ionic Compounds Brittle And Metals Malleable.
From www.slideshare.net
Chemistry Chp 7 Ionic And Metallic Bonding PowerPoint Why Are Ionic Compounds Brittle And Metals Malleable A metal behaves as an array of metal. Ionic compounds are generally hard, but brittle. Your starting assumption that ionic compounds are frequently brittle is misleading. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Gold, silver, aluminum, iron, and copper are malleable. Ionic compounds are generally hard, but. Why Are Ionic Compounds Brittle And Metals Malleable.