Ignition Test Chemistry . Na2s, and nax (x cl, br, i). A copper wire is dipped. — ignition test to identify aromatic and aliphatic compounds ii organic chemistry practical. This test identifies the compound by observing how the sample burns. permanganate test for unsaturation (baeyer test): the beilstein test confirms the presence of a halogen in solution, although it does not distinguish between chlorine, bromine, or iodine. — this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. — preliminary tests: loss on ignition is a method used within inorganic analytical chemistry. loss on ignition is a test used in inorganic analytical chemistry, particularly in the analysis of minerals. — you are correct and in that case, you can use the ignition test. — first year ug experiment of organic chemistry of iit kgp demonstrated. How do you account for the observed. — the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. this experiment aims to differentiate the intrinsic physical and the chemical properties of hydrocarbons, and to.
from www.linstitute.net
Aqueous permanganate rapidly oxidizes double and triple bonds while. — an ignition test in chemistry is a method used to determine the conditions under which a substance will ignite. — first year ug experiment of organic chemistry of iit kgp demonstrated. — preliminary tests: A copper wire is dipped. — ignition test to identify aromatic and aliphatic compounds ii organic chemistry practical. loss on ignition is a test used in inorganic analytical chemistry, particularly in the analysis of minerals. 331, will give you an indication as to whether or not your unknown compound is aromatic. — this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. The premise is that heat.
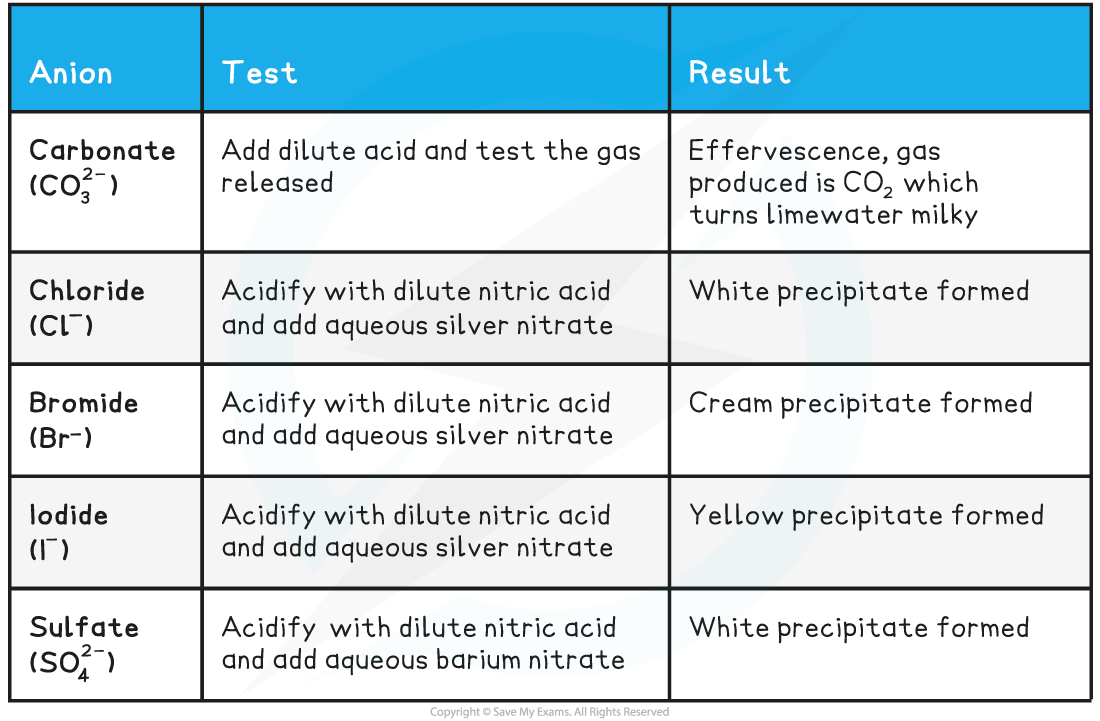
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:2.7.4 Tests for Anions
Ignition Test Chemistry 331, will give you an indication as to whether or not your unknown compound is aromatic. What element is indicated by the residue formed in the charring test? The premise is that heat. — preliminary tests: — the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. — first year ug experiment of organic chemistry of iit kgp demonstrated. the ignition test means heating a substance at a particular temperature. — this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. the beilstein test confirms the presence of a halogen in solution, although it does not distinguish between chlorine, bromine, or iodine. many liquids require an ignition source (a spark, match, or flame) in order for their vapors to catch on fire, a property often. General scheme of analysis a. loss on ignition is a test used in inorganic analytical chemistry, particularly in the analysis of minerals. A copper wire is dipped. loss on ignition is a method used within inorganic analytical chemistry. permanganate test for unsaturation (baeyer test): The practice consists of ‘igniting’ (vigorous heating) a sample at a designated temperature which enables volatile substances within the sample material to escape, until the mass of the sample ceases to change.
From www.researchgate.net
Typical result of ignition test. Download Scientific Diagram Ignition Test Chemistry How do you account for the observed. — ignition test to identify aromatic and aliphatic compounds ii organic chemistry practical. Place a small amount of compound on a spatula. — the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. permanganate test for unsaturation (baeyer test): many. Ignition Test Chemistry.
From flammabilitytestingequipment.com
ISO 871 / ASTM D1929 Plastic Ignition Temperature Testing Equipment Ignition Test Chemistry — the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. Na2s, and nax (x cl, br, i). — preliminary tests: ignition test the ignition test, found on p. the ignition test means heating a substance at a particular temperature. tests for unsaturation can be used. Ignition Test Chemistry.
From www.youtube.com
Organic chemistry ignition test of organic compounds(in hindi Ignition Test Chemistry — first year ug experiment of organic chemistry of iit kgp demonstrated. — the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. How do you account for the observed. the ignition test means heating a substance at a particular temperature. Place a small amount of compound on. Ignition Test Chemistry.
From www.slideserve.com
PPT Identification of an Unknown OxygenContaining Compound Ignition Test Chemistry — in the course of the heating and ignition tests, the thermal decomposition products are identified by sensing or. the beilstein test confirms the presence of a halogen in solution, although it does not distinguish between chlorine, bromine, or iodine. The practice consists of ‘igniting’ (vigorous heating) a sample at a designated temperature which enables volatile substances within. Ignition Test Chemistry.
From s148.photobucket.com
Ignition System Testing.jpg Photo by Wolfmansbrudda Photobucket Ignition Test Chemistry permanganate test for unsaturation (baeyer test): A copper wire is dipped. How do you account for the observed. the beilstein test confirms the presence of a halogen in solution, although it does not distinguish between chlorine, bromine, or iodine. In these tests, one can note all the physical characteristics of the compound that. What element is indicated by. Ignition Test Chemistry.
From appliedionsystems.com
First Ignition Test Prep 1 Applied Ion Systems Ignition Test Chemistry The practice consists of ‘igniting’ (vigorous heating) a sample at a designated temperature which enables volatile substances within the sample material to escape, until the mass of the sample ceases to change. Na2s, and nax (x cl, br, i). General scheme of analysis a. 331, will give you an indication as to whether or not your unknown compound is aromatic.. Ignition Test Chemistry.
From brainly.in
what is ignition test in chemistry??? Brainly.in Ignition Test Chemistry — ignition test to identify aromatic and aliphatic compounds ii organic chemistry practical. permanganate test for unsaturation (baeyer test): The practice consists of ‘igniting’ (vigorous heating) a sample at a designated temperature which enables volatile substances within the sample material to escape, until the mass of the sample ceases to change. 331, will give you an indication as. Ignition Test Chemistry.
From fixenginevicolininq.z22.web.core.windows.net
Test An Ignition Coil Ignition Test Chemistry How do you account for the observed. the ignition test involves a procedure in which a drop or two of a liquid or about 50 mg of a solid is heated gently on a small. Aqueous permanganate rapidly oxidizes double and triple bonds while. loss on ignition is a method used within inorganic analytical chemistry. 331, will give. Ignition Test Chemistry.
From www.autozone.com
Repair Guides Electronic Ignition Diagnosis And Testing Ignition Test Chemistry 331, will give you an indication as to whether or not your unknown compound is aromatic. — an ignition test in chemistry is a method used to determine the conditions under which a substance will ignite. loss on ignition is a test used in inorganic analytical chemistry, particularly in the analysis of minerals. the ignition test involves. Ignition Test Chemistry.
From membraneworks.com.au
Loss on Ignition Testing Membrane Works Ignition Test Chemistry — ignition test to identify aromatic and aliphatic compounds ii organic chemistry practical. tests for unsaturation can be used to identify the double and triple bonds present in the organic compound. A copper wire is dipped. ignition test the ignition test, found on p. In these tests, one can note all the physical characteristics of the compound. Ignition Test Chemistry.
From axleaddict.com
How to Test an Ignition Coil AxleAddict Ignition Test Chemistry This test identifies the compound by observing how the sample burns. many liquids require an ignition source (a spark, match, or flame) in order for their vapors to catch on fire, a property often. — this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises.. Ignition Test Chemistry.
From chemistry.com.pk
Metal Ion Flame Test Colours [Infographic] Ignition Test Chemistry — you are correct and in that case, you can use the ignition test. What element is indicated by the residue formed in the charring test? 331, will give you an indication as to whether or not your unknown compound is aromatic. — ignition test to identify aromatic and aliphatic compounds ii organic chemistry practical. loss on. Ignition Test Chemistry.
From appliedionsystems.com
First Ignition Test Prep 2 Applied Ion Systems Ignition Test Chemistry — the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. this experiment aims to differentiate the intrinsic physical and the chemical properties of hydrocarbons, and to. Na2s, and nax (x cl, br, i). 331, will give you an indication as to whether or not your unknown compound is. Ignition Test Chemistry.
From www.sartorius.com
Residue on Ignition Application Highlight Sartorius Ignition Test Chemistry — ignition test to identify aromatic and aliphatic compounds ii organic chemistry practical. — this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. The premise is that heat. Place a small amount of compound on a spatula. How do you account for the observed.. Ignition Test Chemistry.
From www.youtube.com
Ignition Test to identify Aromatic and Aliphatic Compounds II Organic Ignition Test Chemistry — you are correct and in that case, you can use the ignition test. — an ignition test in chemistry is a method used to determine the conditions under which a substance will ignite. loss on ignition is a test used in inorganic analytical chemistry, particularly in the analysis of minerals. — this page describes how. Ignition Test Chemistry.
From studylib.net
FLAME TEST LAB PROCEDURE Ignition Test Chemistry This test identifies the compound by observing how the sample burns. The premise is that heat. General scheme of analysis a. permanganate test for unsaturation (baeyer test): — in the course of the heating and ignition tests, the thermal decomposition products are identified by sensing or. — preliminary tests: 331, will give you an indication as to. Ignition Test Chemistry.
From screlektroniks.com
Test Machine for HighCurrent Arc Ignition Test (UL 746A) Ignition Test Chemistry In these tests, one can note all the physical characteristics of the compound that. What element is indicated by the residue formed in the charring test? — this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. permanganate test for unsaturation (baeyer test): 331, will. Ignition Test Chemistry.
From axleaddict.com
How to Test an Ignition Coil AxleAddict Ignition Test Chemistry — preliminary tests: A copper wire is dipped. Place a small amount of compound on a spatula. tests for unsaturation can be used to identify the double and triple bonds present in the organic compound. the ignition test means heating a substance at a particular temperature. In these tests, one can note all the physical characteristics of. Ignition Test Chemistry.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:2.7.4 Tests for Anions Ignition Test Chemistry — the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. permanganate test for unsaturation (baeyer test): — preliminary tests: Aqueous permanganate rapidly oxidizes double and triple bonds while. many liquids require an ignition source (a spark, match, or flame) in order for their vapors to catch. Ignition Test Chemistry.
From melscience.com
"Selfignition" experiment MEL Chemistry Ignition Test Chemistry What element is indicated by the residue formed in the charring test? In these tests, one can note all the physical characteristics of the compound that. this experiment aims to differentiate the intrinsic physical and the chemical properties of hydrocarbons, and to. — an ignition test in chemistry is a method used to determine the conditions under which. Ignition Test Chemistry.
From exobygfvi.blob.core.windows.net
Is A Flame Test A Chemical Change at Tim Crow blog Ignition Test Chemistry the ignition test involves a procedure in which a drop or two of a liquid or about 50 mg of a solid is heated gently on a small. Na2s, and nax (x cl, br, i). the ignition test means heating a substance at a particular temperature. tests for unsaturation can be used to identify the double and. Ignition Test Chemistry.
From www.alamy.com
Scientists make a chemical test that results in ignition Stock Video Ignition Test Chemistry — ignition test to identify aromatic and aliphatic compounds ii organic chemistry practical. — an ignition test in chemistry is a method used to determine the conditions under which a substance will ignite. — preliminary tests: How do you account for the observed. ignition test the ignition test, found on p. — the flame test. Ignition Test Chemistry.
From imgbin.com
Ignition90 Chemistry Chemical Test PNG, Clipart, Artwork, Body Jewelry Ignition Test Chemistry tests for unsaturation can be used to identify the double and triple bonds present in the organic compound. loss on ignition is a method used within inorganic analytical chemistry. The practice consists of ‘igniting’ (vigorous heating) a sample at a designated temperature which enables volatile substances within the sample material to escape, until the mass of the sample. Ignition Test Chemistry.
From www.researchgate.net
Ignition test results of the polypropylene panels using ignition Ignition Test Chemistry many liquids require an ignition source (a spark, match, or flame) in order for their vapors to catch on fire, a property often. — ignition test to identify aromatic and aliphatic compounds ii organic chemistry practical. — the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. . Ignition Test Chemistry.
From www.mdpi.com
Metals Free FullText Comparative Study on Combustion Behavior of Ignition Test Chemistry How do you account for the observed. Aqueous permanganate rapidly oxidizes double and triple bonds while. — preliminary tests: this experiment aims to differentiate the intrinsic physical and the chemical properties of hydrocarbons, and to. — you are correct and in that case, you can use the ignition test. — this page describes how to perform. Ignition Test Chemistry.
From www.youtube.com
How to Solve Ignition Problems with MSD’s Universal Ignition Tester Ignition Test Chemistry — ignition test to identify aromatic and aliphatic compounds ii organic chemistry practical. — this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. loss on ignition is a method used within inorganic analytical chemistry. Aqueous permanganate rapidly oxidizes double and triple bonds while.. Ignition Test Chemistry.
From www.youtube.com
HOW TO PERFORM RESIDUE ON IGNITION TEST IN QUALITY CONTROL? YouTube Ignition Test Chemistry How do you account for the observed. loss on ignition is a test used in inorganic analytical chemistry, particularly in the analysis of minerals. This test identifies the compound by observing how the sample burns. General scheme of analysis a. this experiment aims to differentiate the intrinsic physical and the chemical properties of hydrocarbons, and to. —. Ignition Test Chemistry.
From studylib.net
Secondary Ignition Testing Ignition Test Chemistry — preliminary tests: — ignition test to identify aromatic and aliphatic compounds ii organic chemistry practical. — you are correct and in that case, you can use the ignition test. — an ignition test in chemistry is a method used to determine the conditions under which a substance will ignite. 331, will give you an indication. Ignition Test Chemistry.
From repairfixfriziranesd6.z13.web.core.windows.net
How To Check Ignition Coil Resistance Ignition Test Chemistry The premise is that heat. Na2s, and nax (x cl, br, i). General scheme of analysis a. — you are correct and in that case, you can use the ignition test. — first year ug experiment of organic chemistry of iit kgp demonstrated. — in the course of the heating and ignition tests, the thermal decomposition products. Ignition Test Chemistry.
From www.slideserve.com
PPT Lecture No. 04 PowerPoint Presentation, free download ID364248 Ignition Test Chemistry This test identifies the compound by observing how the sample burns. — the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. — first year ug experiment of organic chemistry of iit kgp demonstrated. What element is indicated by the residue formed in the charring test? the ignition. Ignition Test Chemistry.
From www.thoughtco.com
How to Do a Flame Test for Qualitative Analysis Ignition Test Chemistry many liquids require an ignition source (a spark, match, or flame) in order for their vapors to catch on fire, a property often. In these tests, one can note all the physical characteristics of the compound that. 331, will give you an indication as to whether or not your unknown compound is aromatic. — in the course of. Ignition Test Chemistry.
From www.indiamart.com
Ignition Test at best price in New Delhi by Mangal Instrumentation ID Ignition Test Chemistry this experiment aims to differentiate the intrinsic physical and the chemical properties of hydrocarbons, and to. — preliminary tests: — first year ug experiment of organic chemistry of iit kgp demonstrated. tests for unsaturation can be used to identify the double and triple bonds present in the organic compound. Aqueous permanganate rapidly oxidizes double and triple. Ignition Test Chemistry.
From www.youtube.com
Ignition! A Chemistry Book Review YouTube Ignition Test Chemistry — in the course of the heating and ignition tests, the thermal decomposition products are identified by sensing or. Aqueous permanganate rapidly oxidizes double and triple bonds while. — the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. Place a small amount of compound on a spatula. . Ignition Test Chemistry.
From byjus.com
What is an ignition test in Chemistry? Ignition Test Chemistry The premise is that heat. many liquids require an ignition source (a spark, match, or flame) in order for their vapors to catch on fire, a property often. this experiment aims to differentiate the intrinsic physical and the chemical properties of hydrocarbons, and to. Na2s, and nax (x cl, br, i). How do you account for the observed.. Ignition Test Chemistry.
From www.holley.com
MSD 89973 Race Ignition Test Tool Ignition Test Chemistry this experiment aims to differentiate the intrinsic physical and the chemical properties of hydrocarbons, and to. General scheme of analysis a. The premise is that heat. The practice consists of ‘igniting’ (vigorous heating) a sample at a designated temperature which enables volatile substances within the sample material to escape, until the mass of the sample ceases to change. 331,. Ignition Test Chemistry.