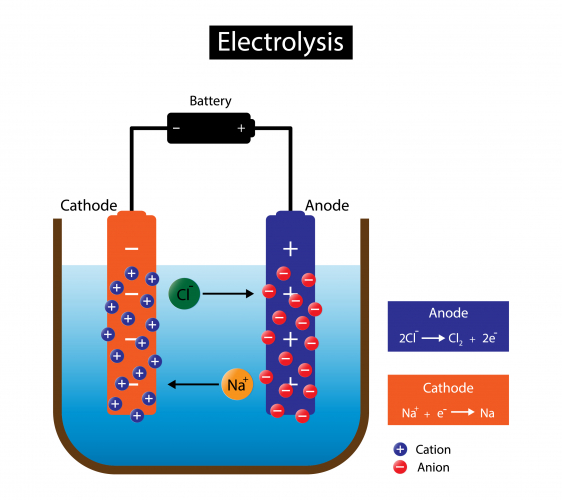
What Electrodes Are Used In Electrolysis . electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in. These electrodes are often made of an. Ionic compounds conduct electricity when. in an electrolytic cell, however, the opposite process, called electrolysis, occurs: electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification. reactive metals are extracted from their ores using electrolysis. two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. Free chemistry revision notes on kinetic theory. electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. what are electrolytes and what happens in electrolysis? An external voltage is applied to drive a. Reactive metals are extracted from their ores using.
from www.edplace.com
These electrodes are often made of an. electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in. Reactive metals are extracted from their ores using. electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. in an electrolytic cell, however, the opposite process, called electrolysis, occurs: two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. what are electrolytes and what happens in electrolysis? reactive metals are extracted from their ores using electrolysis. Free chemistry revision notes on kinetic theory.
Understand How Electrolysis Works Worksheet EdPlace
What Electrodes Are Used In Electrolysis two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. Free chemistry revision notes on kinetic theory. An external voltage is applied to drive a. in an electrolytic cell, however, the opposite process, called electrolysis, occurs: electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in. reactive metals are extracted from their ores using electrolysis. Reactive metals are extracted from their ores using. electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification. two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. what are electrolytes and what happens in electrolysis? These electrodes are often made of an. Ionic compounds conduct electricity when. electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode.
From philschatz.com
Electrolysis · Chemistry What Electrodes Are Used In Electrolysis Ionic compounds conduct electricity when. Reactive metals are extracted from their ores using. reactive metals are extracted from their ores using electrolysis. two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. An external voltage is applied to drive a. in an electrolytic cell, however, the opposite process, called electrolysis, occurs: electrolysis is used. What Electrodes Are Used In Electrolysis.
From www.alamy.com
Electrolysis of copper sulphate solution using copper electrodes Stock What Electrodes Are Used In Electrolysis electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in. Free chemistry revision notes on kinetic theory. Ionic compounds conduct electricity when. electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. in an electrolytic cell,. What Electrodes Are Used In Electrolysis.
From chem2u.blogspot.com
chem2U Electrolysis of Copper(II) Sulphate What Electrodes Are Used In Electrolysis in an electrolytic cell, however, the opposite process, called electrolysis, occurs: two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. Free chemistry revision notes on kinetic theory. what are electrolytes and what happens in electrolysis? reactive metals are extracted from their ores using electrolysis. electrolysis is carried out in an electrolytic cell. What Electrodes Are Used In Electrolysis.
From www.slideserve.com
PPT Electrolysis L.O. I know and can use the terms electrolyte What Electrodes Are Used In Electrolysis Reactive metals are extracted from their ores using. electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification. what are electrolytes and what happens in electrolysis? two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. Ionic compounds conduct electricity when. These electrodes are often made of an. An external voltage. What Electrodes Are Used In Electrolysis.
From wisc.pb.unizin.org
D41.4 Electrolysis Chemistry 109 Fall 2021 What Electrodes Are Used In Electrolysis Free chemistry revision notes on kinetic theory. two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when. Reactive metals are extracted from their ores using. An external voltage is applied to drive a. electrolysis is carried out in an electrolytic cell. What Electrodes Are Used In Electrolysis.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download What Electrodes Are Used In Electrolysis electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. An external voltage is applied to drive a. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in. reactive metals are extracted from their ores using. What Electrodes Are Used In Electrolysis.
From courses.lumenlearning.com
Electrolysis Chemistry What Electrodes Are Used In Electrolysis Free chemistry revision notes on kinetic theory. These electrodes are often made of an. electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. what are electrolytes and what happens in electrolysis? electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification. reactive. What Electrodes Are Used In Electrolysis.
From www.slideserve.com
PPT ELECTROLYSIS OF AQUEOUS SOLUTIONS PowerPoint Presentation, free What Electrodes Are Used In Electrolysis two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. Free chemistry revision notes on kinetic theory. Reactive metals are extracted from their ores using. electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification. These electrodes are often made of an. electrolysis is carried out in an electrolytic cell consisting. What Electrodes Are Used In Electrolysis.
From mungfali.com
Electrolysis Process Diagram What Electrodes Are Used In Electrolysis Reactive metals are extracted from their ores using. Free chemistry revision notes on kinetic theory. An external voltage is applied to drive a. two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. in an electrolytic cell, however, the opposite process, called electrolysis, occurs: electrolysis can occur in electrolytic cells by introducing a power supply,. What Electrodes Are Used In Electrolysis.
From owlcation.com
Electrolysis The Way of the Future Owlcation What Electrodes Are Used In Electrolysis An external voltage is applied to drive a. what are electrolytes and what happens in electrolysis? electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. Ionic compounds conduct electricity when. Reactive metals are extracted from their ores using. electrolysis is used extensively in metallurgical processes, such as. What Electrodes Are Used In Electrolysis.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace What Electrodes Are Used In Electrolysis These electrodes are often made of an. in an electrolytic cell, however, the opposite process, called electrolysis, occurs: electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in. electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively. What Electrodes Are Used In Electrolysis.
From www.sciencenewsforstudents.org
Explainer What is an electrode? Science News for Students What Electrodes Are Used In Electrolysis two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. Reactive metals are extracted from their ores using. reactive metals are extracted from their ores using electrolysis. what are electrolytes and what happens in electrolysis? Ionic compounds conduct electricity when. Free chemistry revision notes on kinetic theory. electrolysis is carried out in an electrolytic. What Electrodes Are Used In Electrolysis.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo What Electrodes Are Used In Electrolysis in an electrolytic cell, however, the opposite process, called electrolysis, occurs: Reactive metals are extracted from their ores using. These electrodes are often made of an. what are electrolytes and what happens in electrolysis? Free chemistry revision notes on kinetic theory. An external voltage is applied to drive a. electrolysis can occur in electrolytic cells by introducing. What Electrodes Are Used In Electrolysis.
From www.vrogue.co
Introduction To Electrolysis Redox Reactions And Elec vrogue.co What Electrodes Are Used In Electrolysis electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in. An external voltage is applied to drive a. Free chemistry revision notes on kinetic theory. Ionic compounds conduct electricity when. in an electrolytic cell, however, the opposite process, called electrolysis, occurs: electrolysis is used extensively. What Electrodes Are Used In Electrolysis.
From brilliant.org
Electrolytic Cells and Electrolysis Brilliant Math & Science Wiki What Electrodes Are Used In Electrolysis These electrodes are often made of an. electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification. Ionic compounds conduct electricity when. reactive metals are extracted from their ores using electrolysis. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in.. What Electrodes Are Used In Electrolysis.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry What Electrodes Are Used In Electrolysis reactive metals are extracted from their ores using electrolysis. two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. Free chemistry revision notes on kinetic theory. An external voltage is applied to drive a. These electrodes are often made of an. electrolysis is carried out in an electrolytic cell consisting of a positively charged anode. What Electrodes Are Used In Electrolysis.
From socratic.org
Electrolysis Chemistry Socratic What Electrodes Are Used In Electrolysis Ionic compounds conduct electricity when. An external voltage is applied to drive a. electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies. What Electrodes Are Used In Electrolysis.
From dxoargcof.blob.core.windows.net
Electrodes Electrolysis Experiment at Cindy Hopson blog What Electrodes Are Used In Electrolysis two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. These electrodes are often made of an. electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. Reactive metals are extracted from their ores using. Ionic compounds conduct electricity when. An external voltage is applied to drive. What Electrodes Are Used In Electrolysis.
From fixlibrarylorierik8.z14.web.core.windows.net
Cathode Charge In Electrolysis What Electrodes Are Used In Electrolysis reactive metals are extracted from their ores using electrolysis. in an electrolytic cell, however, the opposite process, called electrolysis, occurs: An external voltage is applied to drive a. These electrodes are often made of an. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in.. What Electrodes Are Used In Electrolysis.
From enginelibsaprozoic.z21.web.core.windows.net
What Happens At The Cathode In Electrolysis What Electrodes Are Used In Electrolysis reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when. Reactive metals are extracted from their ores using. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in. These electrodes are often made of an. electrolysis is used extensively in metallurgical. What Electrodes Are Used In Electrolysis.
From byjus.com
In electrolysis what are the positive and negative electrodes known as?? What Electrodes Are Used In Electrolysis electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. Free chemistry revision notes on kinetic theory. what are electrolytes and what happens in electrolysis? electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in. Ionic. What Electrodes Are Used In Electrolysis.
From wiringfixmashedpatatasz3.z14.web.core.windows.net
Cathode Charge In Electrolysis What Electrodes Are Used In Electrolysis two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. what are electrolytes and what happens in electrolysis? electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification. Reactive metals are extracted from their ores using. An external voltage is applied to drive a. electrolysis is carried out in an. What Electrodes Are Used In Electrolysis.
From shop.wf-education.com
Understanding Electrolysis What Electrodes Are Used In Electrolysis Reactive metals are extracted from their ores using. Ionic compounds conduct electricity when. These electrodes are often made of an. Free chemistry revision notes on kinetic theory. what are electrolytes and what happens in electrolysis? reactive metals are extracted from their ores using electrolysis. electrolysis is carried out in an electrolytic cell consisting of a positively charged. What Electrodes Are Used In Electrolysis.
From keystagewiki.com
Electrolysis Key Stage Wiki What Electrodes Are Used In Electrolysis two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. reactive metals are extracted from their ores using electrolysis. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in. Reactive metals are extracted from their ores using. electrolysis is used extensively in. What Electrodes Are Used In Electrolysis.
From studycopesettic.z21.web.core.windows.net
Inert Electrodes Gcse What Electrodes Are Used In Electrolysis in an electrolytic cell, however, the opposite process, called electrolysis, occurs: electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification. Free chemistry revision notes on kinetic theory. two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. Reactive metals are extracted from their ores using. These electrodes are often made. What Electrodes Are Used In Electrolysis.
From circuitagolopetzin.z4.web.core.windows.net
What Happens At The Cathode In Electrolysis What Electrodes Are Used In Electrolysis Ionic compounds conduct electricity when. An external voltage is applied to drive a. what are electrolytes and what happens in electrolysis? reactive metals are extracted from their ores using electrolysis. These electrodes are often made of an. electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. . What Electrodes Are Used In Electrolysis.
From fixlibrarycipponiih.z13.web.core.windows.net
What Happens At The Cathode In Electrolysis What Electrodes Are Used In Electrolysis An external voltage is applied to drive a. Reactive metals are extracted from their ores using. what are electrolytes and what happens in electrolysis? Ionic compounds conduct electricity when. These electrodes are often made of an. Free chemistry revision notes on kinetic theory. reactive metals are extracted from their ores using electrolysis. electrolysis can occur in electrolytic. What Electrodes Are Used In Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID297961 What Electrodes Are Used In Electrolysis what are electrolytes and what happens in electrolysis? Reactive metals are extracted from their ores using. reactive metals are extracted from their ores using electrolysis. electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification. Ionic compounds conduct electricity when. Free chemistry revision notes on kinetic theory. in an electrolytic cell, however,. What Electrodes Are Used In Electrolysis.
From www.youtube.com
6 Different Types of Electrodes & their Reactions in Electrochemistry What Electrodes Are Used In Electrolysis electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. in an electrolytic cell, however, the opposite process, called electrolysis, occurs: two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. reactive metals are extracted from their ores using electrolysis. Free chemistry revision notes on. What Electrodes Are Used In Electrolysis.
From www.youtube.com
Electrolysis of Copper Sulphate YouTube What Electrodes Are Used In Electrolysis Free chemistry revision notes on kinetic theory. electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification. An external voltage is applied to drive a. Reactive metals are extracted from their ores using. reactive metals are extracted from their ores using electrolysis. two electrical conductors (electrodes) are immersed in the liquid to be. What Electrodes Are Used In Electrolysis.
From dxoargcof.blob.core.windows.net
Electrodes Electrolysis Experiment at Cindy Hopson blog What Electrodes Are Used In Electrolysis in an electrolytic cell, however, the opposite process, called electrolysis, occurs: electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. reactive metals are extracted from their ores using electrolysis. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force. What Electrodes Are Used In Electrolysis.
From fixlibraryselfinsig3q.z14.web.core.windows.net
What Forms At The Cathode During Electrolysis What Electrodes Are Used In Electrolysis These electrodes are often made of an. what are electrolytes and what happens in electrolysis? electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification. Free chemistry revision notes on kinetic theory. electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. in. What Electrodes Are Used In Electrolysis.
From spmscience.blog.onlinetuition.com.my
Electrolysis SPM Science What Electrodes Are Used In Electrolysis Ionic compounds conduct electricity when. An external voltage is applied to drive a. electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. Free chemistry revision notes on kinetic theory. electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification. electrolysis can occur in. What Electrodes Are Used In Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk What Electrodes Are Used In Electrolysis An external voltage is applied to drive a. electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. two electrical conductors (electrodes) are immersed in the liquid to be electrolyzed. in an electrolytic cell, however, the opposite process, called electrolysis, occurs: electrolysis can occur in electrolytic cells. What Electrodes Are Used In Electrolysis.
From chem.libretexts.org
11.7 Electrolysis Chemistry LibreTexts What Electrodes Are Used In Electrolysis in an electrolytic cell, however, the opposite process, called electrolysis, occurs: what are electrolytes and what happens in electrolysis? electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in. These electrodes are often made of an. electrolysis is used extensively in metallurgical processes, such. What Electrodes Are Used In Electrolysis.