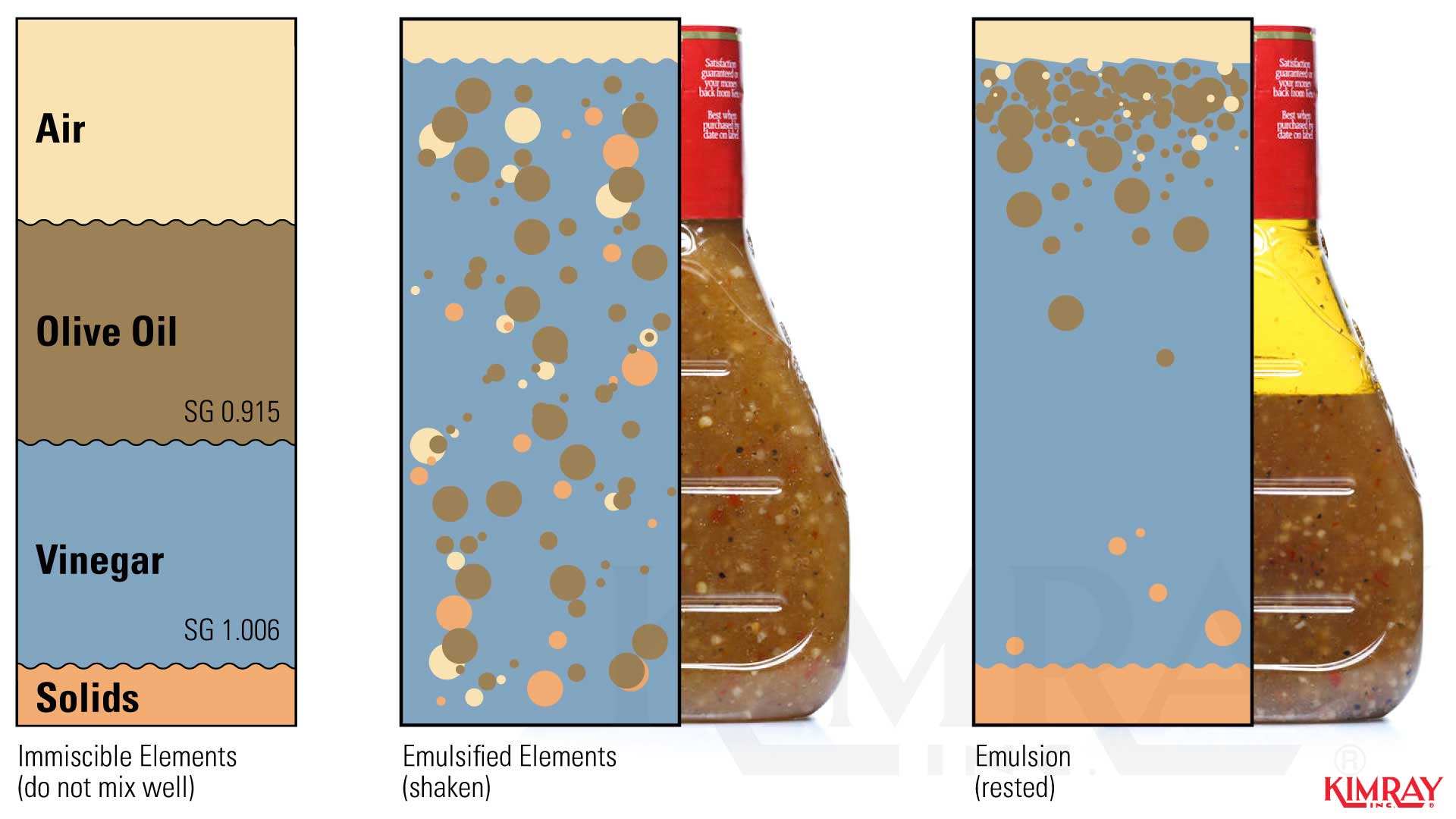
Why Does An Emulsion Separate . Separation of emulsions methods normally used to break emulsions are: The kinetic stability of an emulsion originates from an activation energy (δg ⁎) that must be overcome before the emulsion can. If an emulsion is formed because the two layers have similar densities, try to alter the density of each layer to make them more different. To help clarify an emulsion, try to decrease the. Emulsions are formed by agitating two immiscible liquids, such as oil and water, in the presence of an emulsifier. Emulsions are colloids, which are homogeneous mixtures consisting of particles larger than molecules that scatter light, but are small enough that. Emulsions are thermodynamically unstable as both the dispersed and continuous phases can revert as separate phases, oil, and water, by fusion or the coalescing of. Unstable emulsions can be separated into water and oil over an extended period of time. Emulsifiers can be proteins, phospholipids, or even nanoparticles. There are two primary types of emulsions: Gravity settling—settling of emulsions is more rapid when the drop size.
from kimray.com
Separation of emulsions methods normally used to break emulsions are: If an emulsion is formed because the two layers have similar densities, try to alter the density of each layer to make them more different. To help clarify an emulsion, try to decrease the. Emulsions are formed by agitating two immiscible liquids, such as oil and water, in the presence of an emulsifier. Emulsions are colloids, which are homogeneous mixtures consisting of particles larger than molecules that scatter light, but are small enough that. Emulsions are thermodynamically unstable as both the dispersed and continuous phases can revert as separate phases, oil, and water, by fusion or the coalescing of. Gravity settling—settling of emulsions is more rapid when the drop size. There are two primary types of emulsions: Unstable emulsions can be separated into water and oil over an extended period of time. Emulsifiers can be proteins, phospholipids, or even nanoparticles.
6 Ways to Separate an Oil and Water Emulsion Kimray
Why Does An Emulsion Separate The kinetic stability of an emulsion originates from an activation energy (δg ⁎) that must be overcome before the emulsion can. The kinetic stability of an emulsion originates from an activation energy (δg ⁎) that must be overcome before the emulsion can. Gravity settling—settling of emulsions is more rapid when the drop size. Emulsifiers can be proteins, phospholipids, or even nanoparticles. To help clarify an emulsion, try to decrease the. If an emulsion is formed because the two layers have similar densities, try to alter the density of each layer to make them more different. Emulsions are colloids, which are homogeneous mixtures consisting of particles larger than molecules that scatter light, but are small enough that. Separation of emulsions methods normally used to break emulsions are: There are two primary types of emulsions: Emulsions are thermodynamically unstable as both the dispersed and continuous phases can revert as separate phases, oil, and water, by fusion or the coalescing of. Emulsions are formed by agitating two immiscible liquids, such as oil and water, in the presence of an emulsifier. Unstable emulsions can be separated into water and oil over an extended period of time.
From www.thoughtco.com
What Is an Emulsion? Definition and Examples Why Does An Emulsion Separate To help clarify an emulsion, try to decrease the. Emulsions are formed by agitating two immiscible liquids, such as oil and water, in the presence of an emulsifier. Emulsions are thermodynamically unstable as both the dispersed and continuous phases can revert as separate phases, oil, and water, by fusion or the coalescing of. The kinetic stability of an emulsion originates. Why Does An Emulsion Separate.
From mavink.com
What Is Emulsion Why Does An Emulsion Separate Unstable emulsions can be separated into water and oil over an extended period of time. Separation of emulsions methods normally used to break emulsions are: Emulsions are colloids, which are homogeneous mixtures consisting of particles larger than molecules that scatter light, but are small enough that. The kinetic stability of an emulsion originates from an activation energy (δg ⁎) that. Why Does An Emulsion Separate.
From sciencenotes.org
What Is an Emulsion? Definition and Examples Why Does An Emulsion Separate Gravity settling—settling of emulsions is more rapid when the drop size. To help clarify an emulsion, try to decrease the. The kinetic stability of an emulsion originates from an activation energy (δg ⁎) that must be overcome before the emulsion can. Unstable emulsions can be separated into water and oil over an extended period of time. Emulsions are formed by. Why Does An Emulsion Separate.
From www.researchgate.net
A schematic diagram of emulsion instability Download Scientific Diagram Why Does An Emulsion Separate Gravity settling—settling of emulsions is more rapid when the drop size. To help clarify an emulsion, try to decrease the. Emulsifiers can be proteins, phospholipids, or even nanoparticles. Emulsions are formed by agitating two immiscible liquids, such as oil and water, in the presence of an emulsifier. There are two primary types of emulsions: Separation of emulsions methods normally used. Why Does An Emulsion Separate.
From www.sciencedoze.com
Emulsions Definition, Preparation, Working, Types, Properties Why Does An Emulsion Separate Unstable emulsions can be separated into water and oil over an extended period of time. The kinetic stability of an emulsion originates from an activation energy (δg ⁎) that must be overcome before the emulsion can. Emulsions are thermodynamically unstable as both the dispersed and continuous phases can revert as separate phases, oil, and water, by fusion or the coalescing. Why Does An Emulsion Separate.
From www.researchgate.net
The formation of an emulsion gel (O/W) and the encapsulation of Why Does An Emulsion Separate Unstable emulsions can be separated into water and oil over an extended period of time. The kinetic stability of an emulsion originates from an activation energy (δg ⁎) that must be overcome before the emulsion can. There are two primary types of emulsions: Emulsifiers can be proteins, phospholipids, or even nanoparticles. Emulsions are thermodynamically unstable as both the dispersed and. Why Does An Emulsion Separate.
From thesciencecore.blogspot.com
Emulsion definition, properties, and examples The Science Core Why Does An Emulsion Separate Unstable emulsions can be separated into water and oil over an extended period of time. Emulsifiers can be proteins, phospholipids, or even nanoparticles. Emulsions are formed by agitating two immiscible liquids, such as oil and water, in the presence of an emulsifier. Separation of emulsions methods normally used to break emulsions are: There are two primary types of emulsions: The. Why Does An Emulsion Separate.
From www.lankem.com
Principles of Emulsification Why Does An Emulsion Separate To help clarify an emulsion, try to decrease the. The kinetic stability of an emulsion originates from an activation energy (δg ⁎) that must be overcome before the emulsion can. If an emulsion is formed because the two layers have similar densities, try to alter the density of each layer to make them more different. Unstable emulsions can be separated. Why Does An Emulsion Separate.
From www.biolinscientific.com
How emulsions form and break? Why Does An Emulsion Separate Emulsions are colloids, which are homogeneous mixtures consisting of particles larger than molecules that scatter light, but are small enough that. Emulsifiers can be proteins, phospholipids, or even nanoparticles. There are two primary types of emulsions: Unstable emulsions can be separated into water and oil over an extended period of time. Emulsions are formed by agitating two immiscible liquids, such. Why Does An Emulsion Separate.
From giozrpndg.blob.core.windows.net
Why Does An Emulsion Separate at Susan Robb blog Why Does An Emulsion Separate Unstable emulsions can be separated into water and oil over an extended period of time. Separation of emulsions methods normally used to break emulsions are: Emulsions are colloids, which are homogeneous mixtures consisting of particles larger than molecules that scatter light, but are small enough that. There are two primary types of emulsions: Emulsions are formed by agitating two immiscible. Why Does An Emulsion Separate.
From summerrain.website
Emulsions What Are They and How Do They Work? Summer Rain Why Does An Emulsion Separate Gravity settling—settling of emulsions is more rapid when the drop size. To help clarify an emulsion, try to decrease the. Emulsions are thermodynamically unstable as both the dispersed and continuous phases can revert as separate phases, oil, and water, by fusion or the coalescing of. There are two primary types of emulsions: Emulsifiers can be proteins, phospholipids, or even nanoparticles.. Why Does An Emulsion Separate.
From www.science-sparks.com
What is an Emulsion? Why Does An Emulsion Separate Emulsions are formed by agitating two immiscible liquids, such as oil and water, in the presence of an emulsifier. Unstable emulsions can be separated into water and oil over an extended period of time. There are two primary types of emulsions: The kinetic stability of an emulsion originates from an activation energy (δg ⁎) that must be overcome before the. Why Does An Emulsion Separate.
From www.researchgate.net
Emulsion formation from oil and water by addition of an emulsifier Why Does An Emulsion Separate Separation of emulsions methods normally used to break emulsions are: Unstable emulsions can be separated into water and oil over an extended period of time. There are two primary types of emulsions: Emulsions are formed by agitating two immiscible liquids, such as oil and water, in the presence of an emulsifier. Gravity settling—settling of emulsions is more rapid when the. Why Does An Emulsion Separate.
From www.youtube.com
What Is An Emulsion & How Does It Work? YouTube Why Does An Emulsion Separate Separation of emulsions methods normally used to break emulsions are: Emulsions are colloids, which are homogeneous mixtures consisting of particles larger than molecules that scatter light, but are small enough that. If an emulsion is formed because the two layers have similar densities, try to alter the density of each layer to make them more different. The kinetic stability of. Why Does An Emulsion Separate.
From giozrpndg.blob.core.windows.net
Why Does An Emulsion Separate at Susan Robb blog Why Does An Emulsion Separate To help clarify an emulsion, try to decrease the. If an emulsion is formed because the two layers have similar densities, try to alter the density of each layer to make them more different. Emulsions are formed by agitating two immiscible liquids, such as oil and water, in the presence of an emulsifier. Emulsifiers can be proteins, phospholipids, or even. Why Does An Emulsion Separate.
From www.mdpi.com
Processes Free FullText The Formation, Stabilization and Why Does An Emulsion Separate Emulsions are thermodynamically unstable as both the dispersed and continuous phases can revert as separate phases, oil, and water, by fusion or the coalescing of. Emulsifiers can be proteins, phospholipids, or even nanoparticles. Separation of emulsions methods normally used to break emulsions are: There are two primary types of emulsions: The kinetic stability of an emulsion originates from an activation. Why Does An Emulsion Separate.
From foodsciencetoolbox.com
Making an Emulsion Food Science Toolbox Why Does An Emulsion Separate Emulsions are colloids, which are homogeneous mixtures consisting of particles larger than molecules that scatter light, but are small enough that. Separation of emulsions methods normally used to break emulsions are: Gravity settling—settling of emulsions is more rapid when the drop size. Emulsions are thermodynamically unstable as both the dispersed and continuous phases can revert as separate phases, oil, and. Why Does An Emulsion Separate.
From giozrpndg.blob.core.windows.net
Why Does An Emulsion Separate at Susan Robb blog Why Does An Emulsion Separate Emulsifiers can be proteins, phospholipids, or even nanoparticles. If an emulsion is formed because the two layers have similar densities, try to alter the density of each layer to make them more different. Separation of emulsions methods normally used to break emulsions are: The kinetic stability of an emulsion originates from an activation energy (δg ⁎) that must be overcome. Why Does An Emulsion Separate.
From solutionpharmacy.in
Preparation of Emulsions Solution Parmacy Why Does An Emulsion Separate Emulsions are formed by agitating two immiscible liquids, such as oil and water, in the presence of an emulsifier. Emulsions are thermodynamically unstable as both the dispersed and continuous phases can revert as separate phases, oil, and water, by fusion or the coalescing of. The kinetic stability of an emulsion originates from an activation energy (δg ⁎) that must be. Why Does An Emulsion Separate.
From journals.sagepub.com
The structure and property of polyacrylonitrilebased microfiltration Why Does An Emulsion Separate To help clarify an emulsion, try to decrease the. Emulsifiers can be proteins, phospholipids, or even nanoparticles. Unstable emulsions can be separated into water and oil over an extended period of time. If an emulsion is formed because the two layers have similar densities, try to alter the density of each layer to make them more different. Gravity settling—settling of. Why Does An Emulsion Separate.
From medhop.weebly.com
Solid emulsion definition chemistry medHop Why Does An Emulsion Separate There are two primary types of emulsions: Emulsions are colloids, which are homogeneous mixtures consisting of particles larger than molecules that scatter light, but are small enough that. The kinetic stability of an emulsion originates from an activation energy (δg ⁎) that must be overcome before the emulsion can. Emulsions are thermodynamically unstable as both the dispersed and continuous phases. Why Does An Emulsion Separate.
From www.lankem.com
Principles of Emulsification Why Does An Emulsion Separate If an emulsion is formed because the two layers have similar densities, try to alter the density of each layer to make them more different. Gravity settling—settling of emulsions is more rapid when the drop size. Separation of emulsions methods normally used to break emulsions are: Emulsions are formed by agitating two immiscible liquids, such as oil and water, in. Why Does An Emulsion Separate.
From www.slideserve.com
PPT EMULSIONS PowerPoint Presentation, free download ID138804 Why Does An Emulsion Separate The kinetic stability of an emulsion originates from an activation energy (δg ⁎) that must be overcome before the emulsion can. If an emulsion is formed because the two layers have similar densities, try to alter the density of each layer to make them more different. Emulsions are colloids, which are homogeneous mixtures consisting of particles larger than molecules that. Why Does An Emulsion Separate.
From www.researchgate.net
Schematic illustration of nanoemulsions with (a) oilinwater emulsions Why Does An Emulsion Separate Separation of emulsions methods normally used to break emulsions are: Gravity settling—settling of emulsions is more rapid when the drop size. To help clarify an emulsion, try to decrease the. Emulsions are formed by agitating two immiscible liquids, such as oil and water, in the presence of an emulsifier. If an emulsion is formed because the two layers have similar. Why Does An Emulsion Separate.
From www.britannica.com
Emulsion Definition & Types Britannica Why Does An Emulsion Separate Unstable emulsions can be separated into water and oil over an extended period of time. Gravity settling—settling of emulsions is more rapid when the drop size. To help clarify an emulsion, try to decrease the. Separation of emulsions methods normally used to break emulsions are: Emulsifiers can be proteins, phospholipids, or even nanoparticles. Emulsions are thermodynamically unstable as both the. Why Does An Emulsion Separate.
From www.youtube.com
6 Ways to Separate an Oil and Water Emulsion [Oil & Gas Industry Basics Why Does An Emulsion Separate Unstable emulsions can be separated into water and oil over an extended period of time. Emulsions are thermodynamically unstable as both the dispersed and continuous phases can revert as separate phases, oil, and water, by fusion or the coalescing of. If an emulsion is formed because the two layers have similar densities, try to alter the density of each layer. Why Does An Emulsion Separate.
From www.slideshare.net
Emulsion Why Does An Emulsion Separate To help clarify an emulsion, try to decrease the. Emulsions are thermodynamically unstable as both the dispersed and continuous phases can revert as separate phases, oil, and water, by fusion or the coalescing of. There are two primary types of emulsions: Unstable emulsions can be separated into water and oil over an extended period of time. Emulsions are formed by. Why Does An Emulsion Separate.
From www.slideserve.com
PPT Emulsion technology PowerPoint Presentation, free download ID Why Does An Emulsion Separate Emulsifiers can be proteins, phospholipids, or even nanoparticles. Unstable emulsions can be separated into water and oil over an extended period of time. To help clarify an emulsion, try to decrease the. The kinetic stability of an emulsion originates from an activation energy (δg ⁎) that must be overcome before the emulsion can. Gravity settling—settling of emulsions is more rapid. Why Does An Emulsion Separate.
From www.researchgate.net
Different mechanisms of emulsion destabilization, depending on the Why Does An Emulsion Separate To help clarify an emulsion, try to decrease the. Unstable emulsions can be separated into water and oil over an extended period of time. Emulsions are thermodynamically unstable as both the dispersed and continuous phases can revert as separate phases, oil, and water, by fusion or the coalescing of. There are two primary types of emulsions: Gravity settling—settling of emulsions. Why Does An Emulsion Separate.
From kimray.com
6 Ways to Separate an Oil and Water Emulsion Kimray Why Does An Emulsion Separate Emulsifiers can be proteins, phospholipids, or even nanoparticles. Unstable emulsions can be separated into water and oil over an extended period of time. Emulsions are thermodynamically unstable as both the dispersed and continuous phases can revert as separate phases, oil, and water, by fusion or the coalescing of. To help clarify an emulsion, try to decrease the. Emulsions are formed. Why Does An Emulsion Separate.
From www.youtube.com
Emulsions and types of emulsions in English Emulsions Types of Why Does An Emulsion Separate Emulsions are formed by agitating two immiscible liquids, such as oil and water, in the presence of an emulsifier. Unstable emulsions can be separated into water and oil over an extended period of time. Emulsions are thermodynamically unstable as both the dispersed and continuous phases can revert as separate phases, oil, and water, by fusion or the coalescing of. To. Why Does An Emulsion Separate.
From www.aquaportail.com
Émulsion définition et explications Why Does An Emulsion Separate The kinetic stability of an emulsion originates from an activation energy (δg ⁎) that must be overcome before the emulsion can. Emulsions are formed by agitating two immiscible liquids, such as oil and water, in the presence of an emulsifier. Gravity settling—settling of emulsions is more rapid when the drop size. To help clarify an emulsion, try to decrease the.. Why Does An Emulsion Separate.
From www.researchgate.net
(a−e) Oil−water emulsion separation process. (f and g) Optical Why Does An Emulsion Separate To help clarify an emulsion, try to decrease the. Emulsions are formed by agitating two immiscible liquids, such as oil and water, in the presence of an emulsifier. If an emulsion is formed because the two layers have similar densities, try to alter the density of each layer to make them more different. Gravity settling—settling of emulsions is more rapid. Why Does An Emulsion Separate.
From www.slideserve.com
PPT Emulsions PowerPoint Presentation, free download ID824681 Why Does An Emulsion Separate The kinetic stability of an emulsion originates from an activation energy (δg ⁎) that must be overcome before the emulsion can. Gravity settling—settling of emulsions is more rapid when the drop size. Emulsions are formed by agitating two immiscible liquids, such as oil and water, in the presence of an emulsifier. Emulsifiers can be proteins, phospholipids, or even nanoparticles. There. Why Does An Emulsion Separate.
From www.researchgate.net
Phase separation types observed in the emulsion, (A) liquid single Why Does An Emulsion Separate If an emulsion is formed because the two layers have similar densities, try to alter the density of each layer to make them more different. Emulsions are thermodynamically unstable as both the dispersed and continuous phases can revert as separate phases, oil, and water, by fusion or the coalescing of. Emulsifiers can be proteins, phospholipids, or even nanoparticles. The kinetic. Why Does An Emulsion Separate.