Highest Ksp Value . And what are the k s p units? salt effect (diverse ion effect): 176 rows solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. the ksp of a slightly soluble ionic compound may be simply related to its measured solubility provided the dissolution process. what is solubility product constant (ksp). The k s p value does. the more soluble a substance is, the higher its k s p chemistry value. the value of the constant identifies the degree to which the compound can dissociate in water. How to calculate molar solubility from ksp. learn what the solubility product constant is, how to calculate ksp, and how to calculate molar solubility using the solubility. Check out an example and a few problems with answers. the equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the. Actually, it doesn’t have a unit! Having an opposing effect on the \(k_{sp}\) value compared to the common ion effect,.
from www.chegg.com
what is solubility product constant (ksp). the ksp of a slightly soluble ionic compound may be simply related to its measured solubility provided the dissolution process. And what are the k s p units? Actually, it doesn’t have a unit! the equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the. The k s p value does. Check out an example and a few problems with answers. How to calculate molar solubility from ksp. the more soluble a substance is, the higher its k s p chemistry value. Having an opposing effect on the \(k_{sp}\) value compared to the common ion effect,.
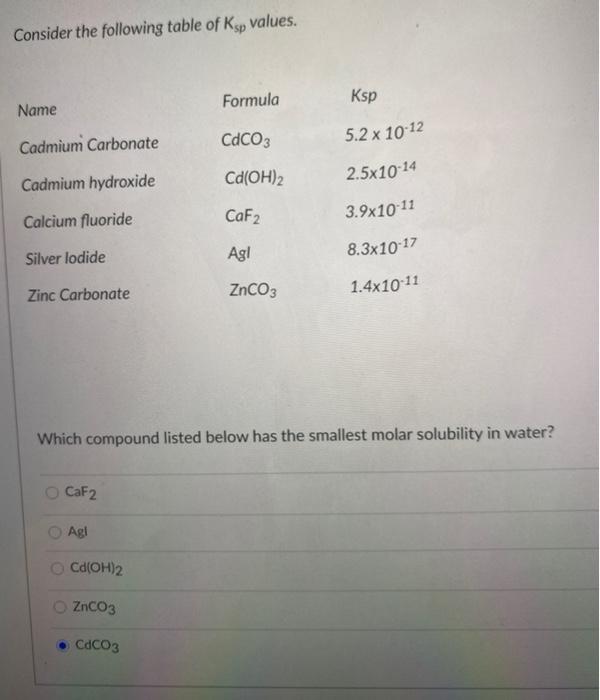
Solved Consider the following table of Ksp values. Formula
Highest Ksp Value Having an opposing effect on the \(k_{sp}\) value compared to the common ion effect,. the value of the constant identifies the degree to which the compound can dissociate in water. Check out an example and a few problems with answers. 176 rows solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. learn what the solubility product constant is, how to calculate ksp, and how to calculate molar solubility using the solubility. the more soluble a substance is, the higher its k s p chemistry value. Having an opposing effect on the \(k_{sp}\) value compared to the common ion effect,. The k s p value does. what is solubility product constant (ksp). And what are the k s p units? the ksp of a slightly soluble ionic compound may be simply related to its measured solubility provided the dissolution process. salt effect (diverse ion effect): the equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the. Actually, it doesn’t have a unit! How to calculate molar solubility from ksp.
From www.slideserve.com
PPT Equilibrium Expression (Keq) PowerPoint Presentation, free Highest Ksp Value And what are the k s p units? Check out an example and a few problems with answers. learn what the solubility product constant is, how to calculate ksp, and how to calculate molar solubility using the solubility. the ksp of a slightly soluble ionic compound may be simply related to its measured solubility provided the dissolution process.. Highest Ksp Value.
From www.numerade.com
SOLVEDDetermine the Ksp value for zinc fluoride; whose maximum Highest Ksp Value what is solubility product constant (ksp). Actually, it doesn’t have a unit! And what are the k s p units? How to calculate molar solubility from ksp. Having an opposing effect on the \(k_{sp}\) value compared to the common ion effect,. the equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the.. Highest Ksp Value.
From www.slideserve.com
PPT Ksp and the Common Ion Effect PowerPoint Presentation ID5978246 Highest Ksp Value what is solubility product constant (ksp). the more soluble a substance is, the higher its k s p chemistry value. The k s p value does. salt effect (diverse ion effect): 176 rows solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. And what are the k s p units?. Highest Ksp Value.
From www.chegg.com
Solved Consider the following table of Ksp values. Name Highest Ksp Value salt effect (diverse ion effect): the equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the. Having an opposing effect on the \(k_{sp}\) value compared to the common ion effect,. Check out an example and a few problems with answers. How to calculate molar solubility from ksp. the more soluble a. Highest Ksp Value.
From www.researchgate.net
Cleaned Kmap (top) and regions with higher KSP value (Φ > 0.5 Highest Ksp Value the equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the. Actually, it doesn’t have a unit! the more soluble a substance is, the higher its k s p chemistry value. what is solubility product constant (ksp). Check out an example and a few problems with answers. The k s p. Highest Ksp Value.
From www.chegg.com
Solved Which of the following will have the highest Ksp Highest Ksp Value learn what the solubility product constant is, how to calculate ksp, and how to calculate molar solubility using the solubility. the ksp of a slightly soluble ionic compound may be simply related to its measured solubility provided the dissolution process. the value of the constant identifies the degree to which the compound can dissociate in water. . Highest Ksp Value.
From www.chegg.com
Solved 5. What does the value of Ksp tell you in terms of Highest Ksp Value Actually, it doesn’t have a unit! The k s p value does. salt effect (diverse ion effect): 176 rows solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. learn what the solubility product constant is, how to calculate ksp, and how to calculate molar solubility using the solubility. Having an opposing. Highest Ksp Value.
From wiki.kerbalspaceprogram.com
Cheat sheet Kerbal Space Program Wiki Highest Ksp Value the more soluble a substance is, the higher its k s p chemistry value. 176 rows solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. Check out an example and a few problems with answers. the equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of. Highest Ksp Value.
From www.youtube.com
Legendary KSP 45 is the Highest DAMAGE SMG COD MOBILE YouTube Highest Ksp Value And what are the k s p units? the more soluble a substance is, the higher its k s p chemistry value. learn what the solubility product constant is, how to calculate ksp, and how to calculate molar solubility using the solubility. Having an opposing effect on the \(k_{sp}\) value compared to the common ion effect,. The k. Highest Ksp Value.
From www.researchgate.net
KSP values for the model examples with hexagonal symmetry. Download Table Highest Ksp Value 176 rows solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. Actually, it doesn’t have a unit! what is solubility product constant (ksp). the ksp of a slightly soluble ionic compound may be simply related to its measured solubility provided the dissolution process. the value of the constant identifies the. Highest Ksp Value.
From www.doubtnut.com
Which has the maximum ksp value Highest Ksp Value The k s p value does. How to calculate molar solubility from ksp. Check out an example and a few problems with answers. the equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the. the ksp of a slightly soluble ionic compound may be simply related to its measured solubility provided the. Highest Ksp Value.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID5723241 Highest Ksp Value How to calculate molar solubility from ksp. the value of the constant identifies the degree to which the compound can dissociate in water. what is solubility product constant (ksp). And what are the k s p units? The k s p value does. Having an opposing effect on the \(k_{sp}\) value compared to the common ion effect,. . Highest Ksp Value.
From www.youtube.com
Lecture 7 Ksp Common Ion Calculation YouTube Highest Ksp Value what is solubility product constant (ksp). learn what the solubility product constant is, how to calculate ksp, and how to calculate molar solubility using the solubility. The k s p value does. salt effect (diverse ion effect): Having an opposing effect on the \(k_{sp}\) value compared to the common ion effect,. the value of the constant. Highest Ksp Value.
From www.chegg.com
Solved Consider the following table of Ksp values. Formula Highest Ksp Value Having an opposing effect on the \(k_{sp}\) value compared to the common ion effect,. Check out an example and a few problems with answers. the ksp of a slightly soluble ionic compound may be simply related to its measured solubility provided the dissolution process. what is solubility product constant (ksp). the value of the constant identifies the. Highest Ksp Value.
From www.slideserve.com
PPT Unit 7 Chpt 16 Solubility equilibria and Quantitative Highest Ksp Value Actually, it doesn’t have a unit! Having an opposing effect on the \(k_{sp}\) value compared to the common ion effect,. learn what the solubility product constant is, how to calculate ksp, and how to calculate molar solubility using the solubility. How to calculate molar solubility from ksp. the value of the constant identifies the degree to which the. Highest Ksp Value.
From z-cm.blogspot.com
Ksp Table Decoration Examples Highest Ksp Value salt effect (diverse ion effect): The k s p value does. learn what the solubility product constant is, how to calculate ksp, and how to calculate molar solubility using the solubility. Actually, it doesn’t have a unit! Having an opposing effect on the \(k_{sp}\) value compared to the common ion effect,. How to calculate molar solubility from ksp.. Highest Ksp Value.
From www.chegg.com
Solved 1. Considering the following table of Ksp values. Highest Ksp Value 176 rows solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. salt effect (diverse ion effect): Check out an example and a few problems with answers. And what are the k s p units? the more soluble a substance is, the higher its k s p chemistry value. the equilibrium. Highest Ksp Value.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID6260427 Highest Ksp Value The k s p value does. the more soluble a substance is, the higher its k s p chemistry value. How to calculate molar solubility from ksp. 176 rows solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. Having an opposing effect on the \(k_{sp}\) value compared to the common ion effect,.. Highest Ksp Value.
From www.studyxapp.com
5 below is a table of k solubility product constant values consider how Highest Ksp Value How to calculate molar solubility from ksp. Check out an example and a few problems with answers. the equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the. And what are the k s p units? the ksp of a slightly soluble ionic compound may be simply related to its measured solubility. Highest Ksp Value.
From www.slideshare.net
CM4106 Review of Lesson 4 Highest Ksp Value How to calculate molar solubility from ksp. salt effect (diverse ion effect): learn what the solubility product constant is, how to calculate ksp, and how to calculate molar solubility using the solubility. And what are the k s p units? the value of the constant identifies the degree to which the compound can dissociate in water. Check. Highest Ksp Value.
From www.chegg.com
Solved Consider the following table of Ksp values. Highest Ksp Value the ksp of a slightly soluble ionic compound may be simply related to its measured solubility provided the dissolution process. How to calculate molar solubility from ksp. Having an opposing effect on the \(k_{sp}\) value compared to the common ion effect,. the equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the.. Highest Ksp Value.
From www.doubtnut.com
[Odia] Which has the maximum ksp value Highest Ksp Value what is solubility product constant (ksp). learn what the solubility product constant is, how to calculate ksp, and how to calculate molar solubility using the solubility. Actually, it doesn’t have a unit! 176 rows solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. Check out an example and a few problems. Highest Ksp Value.
From www.chegg.com
Solved Table 1 List of Various Ksp Values for Cations at Highest Ksp Value How to calculate molar solubility from ksp. the more soluble a substance is, the higher its k s p chemistry value. what is solubility product constant (ksp). salt effect (diverse ion effect): The k s p value does. Check out an example and a few problems with answers. the ksp of a slightly soluble ionic compound. Highest Ksp Value.
From mavink.com
Ksp Values Chart Highest Ksp Value the equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the. learn what the solubility product constant is, how to calculate ksp, and how to calculate molar solubility using the solubility. the more soluble a substance is, the higher its k s p chemistry value. Actually, it doesn’t have a unit!. Highest Ksp Value.
From specialscaqwe.weebly.com
Ksp Cheat Sheet specialscaqwe Highest Ksp Value Actually, it doesn’t have a unit! the equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the. salt effect (diverse ion effect): 176 rows solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. learn what the solubility product constant is, how to calculate ksp,. Highest Ksp Value.
From www.numerade.com
SOLVED Texts 2. [11 Points] DETAILS Use the given Ksp values to Highest Ksp Value salt effect (diverse ion effect): the ksp of a slightly soluble ionic compound may be simply related to its measured solubility provided the dissolution process. what is solubility product constant (ksp). The k s p value does. the equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the. Having an. Highest Ksp Value.
From mavink.com
Ksp Values Chart Highest Ksp Value Check out an example and a few problems with answers. salt effect (diverse ion effect): Having an opposing effect on the \(k_{sp}\) value compared to the common ion effect,. the value of the constant identifies the degree to which the compound can dissociate in water. How to calculate molar solubility from ksp. the more soluble a substance. Highest Ksp Value.
From slideplayer.com
Lesson 6 The Solubility Product ppt download Highest Ksp Value learn what the solubility product constant is, how to calculate ksp, and how to calculate molar solubility using the solubility. what is solubility product constant (ksp). And what are the k s p units? the more soluble a substance is, the higher its k s p chemistry value. Actually, it doesn’t have a unit! the ksp. Highest Ksp Value.
From ksp3pl.com
KSP Core Values Highest Ksp Value The k s p value does. Having an opposing effect on the \(k_{sp}\) value compared to the common ion effect,. 176 rows solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. learn what the solubility product constant is, how to calculate ksp, and how to calculate molar solubility using the solubility. . Highest Ksp Value.
From quizdbsignalizes.z21.web.core.windows.net
How To Solve Ksp Highest Ksp Value the more soluble a substance is, the higher its k s p chemistry value. How to calculate molar solubility from ksp. Check out an example and a few problems with answers. salt effect (diverse ion effect): Having an opposing effect on the \(k_{sp}\) value compared to the common ion effect,. the equilibrium constant for a dissolution reaction,. Highest Ksp Value.
From www.chemistrylearner.com
Solubility Product Constant (Ksp) Definition and Equation Highest Ksp Value the more soluble a substance is, the higher its k s p chemistry value. the equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the. learn what the solubility product constant is, how to calculate ksp, and how to calculate molar solubility using the solubility. Check out an example and a. Highest Ksp Value.
From www.slideserve.com
PPT Solubility Equilibria PowerPoint Presentation, free download ID Highest Ksp Value Actually, it doesn’t have a unit! the value of the constant identifies the degree to which the compound can dissociate in water. Having an opposing effect on the \(k_{sp}\) value compared to the common ion effect,. The k s p value does. learn what the solubility product constant is, how to calculate ksp, and how to calculate molar. Highest Ksp Value.
From www.numerade.com
Consider the following table of Ksp values. Name Formula Ksp Cadmium Highest Ksp Value the value of the constant identifies the degree to which the compound can dissociate in water. And what are the k s p units? Actually, it doesn’t have a unit! Check out an example and a few problems with answers. the equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the. . Highest Ksp Value.
From www.sliderbase.com
Solubility Equilibria Presentation Chemistry Highest Ksp Value Actually, it doesn’t have a unit! salt effect (diverse ion effect): The k s p value does. Having an opposing effect on the \(k_{sp}\) value compared to the common ion effect,. the more soluble a substance is, the higher its k s p chemistry value. the ksp of a slightly soluble ionic compound may be simply related. Highest Ksp Value.
From www.scribd.com
KSP chart Hydroxide Cobalt Highest Ksp Value the ksp of a slightly soluble ionic compound may be simply related to its measured solubility provided the dissolution process. learn what the solubility product constant is, how to calculate ksp, and how to calculate molar solubility using the solubility. the more soluble a substance is, the higher its k s p chemistry value. The k s. Highest Ksp Value.