Chlorine + Magnesium Iodide Balanced Equation . Chlorine + magnesium iodide = diiodine + magnesium chloride. Dichlorine + magnesium iodide = magnesium chloride + diiodine. Balance a chemical equation when given the unbalanced equation. This is a bit of a simplification of the reactions but the. Magnesium chloride and iodine with form an equilibrium. In this example the electrons are shown as dots and crosses. It is sometimes convenient to use fractions instead of integers as intermediate coefficients in the process of balancing a chemical equation. Cl + mgi2 = i2 + mgcl2 is a single displacement (substitution). 1 cl 2 + 1 mgi 2 = 1 mgcl 2 + 1 i 2 for each element, we. Explain the role of the law of conservation of mass in a chemical reaction. Chlorine is in group 7 of the periodic table. Let's balance this equation using the inspection method. Cl + mgi2 = mgcl2 + i is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one mole of aqueous. Cl2 + mgi2 = mgcl2 + i2 is a single displacement. First, we set all coefficients to 1:
from www.numerade.com
Let's balance this equation using the inspection method. 1 cl 2 + 1 mgi 2 = 1 mgcl 2 + 1 i 2 for each element, we. Cl + mgi2 = mgcl2 + i is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one mole of aqueous. Chlorine is in group 7 of the periodic table. Dichlorine + magnesium iodide = magnesium chloride + diiodine. In this example the electrons are shown as dots and crosses. It is sometimes convenient to use fractions instead of integers as intermediate coefficients in the process of balancing a chemical equation. Explain the role of the law of conservation of mass in a chemical reaction. Balance a chemical equation when given the unbalanced equation. This is a bit of a simplification of the reactions but the.
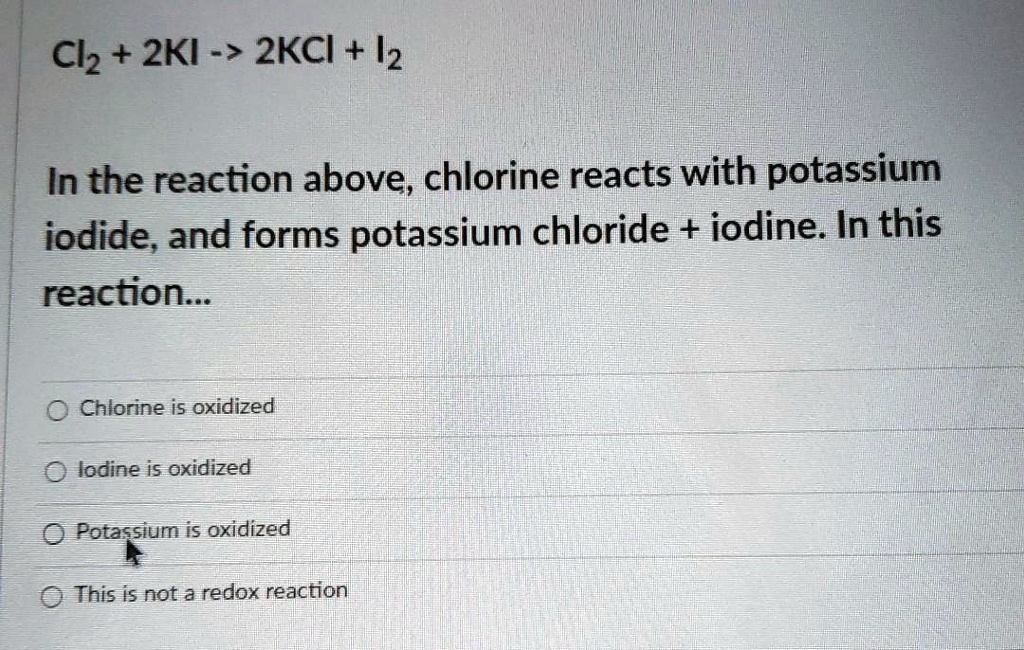
SOLVED Cl2 + 2KI > 2KCl + I2 In the reaction above, chlorine reacts
Chlorine + Magnesium Iodide Balanced Equation Cl + mgi2 = mgcl2 + i is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one mole of aqueous. Cl + mgi2 = mgcl2 + i is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one mole of aqueous. Explain the role of the law of conservation of mass in a chemical reaction. Chlorine + magnesium iodide = diiodine + magnesium chloride. In this example the electrons are shown as dots and crosses. Let's balance this equation using the inspection method. It is sometimes convenient to use fractions instead of integers as intermediate coefficients in the process of balancing a chemical equation. Dichlorine + magnesium iodide = magnesium chloride + diiodine. Magnesium chloride and iodine with form an equilibrium. Chlorine is in group 7 of the periodic table. Cl2 + mgi2 = mgcl2 + i2 is a single displacement. Balance a chemical equation when given the unbalanced equation. 1 cl 2 + 1 mgi 2 = 1 mgcl 2 + 1 i 2 for each element, we. First, we set all coefficients to 1: Cl + mgi2 = i2 + mgcl2 is a single displacement (substitution). This is a bit of a simplification of the reactions but the.
From www.bharatagritech.com
What Is A Chemical Equation For The Reaction Between, 52 OFF Chlorine + Magnesium Iodide Balanced Equation Cl + mgi2 = mgcl2 + i is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one mole of aqueous. Cl + mgi2 = i2 + mgcl2 is a single displacement (substitution). Explain the role of the law of conservation of mass in a chemical reaction. Let's balance this equation using the inspection method. Balance. Chlorine + Magnesium Iodide Balanced Equation.
From signalticket9.pythonanywhere.com
First Class Word Equation For Sodium And Chlorine Thermal Energy Chlorine + Magnesium Iodide Balanced Equation Explain the role of the law of conservation of mass in a chemical reaction. Cl + mgi2 = mgcl2 + i is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one mole of aqueous. Magnesium chloride and iodine with form an equilibrium. 1 cl 2 + 1 mgi 2 = 1 mgcl 2 + 1. Chlorine + Magnesium Iodide Balanced Equation.
From byjus.com
Write word equation and balanced chemical equation for the following Chlorine + Magnesium Iodide Balanced Equation Cl + mgi2 = i2 + mgcl2 is a single displacement (substitution). Let's balance this equation using the inspection method. First, we set all coefficients to 1: Cl2 + mgi2 = mgcl2 + i2 is a single displacement. Balance a chemical equation when given the unbalanced equation. In this example the electrons are shown as dots and crosses. It is. Chlorine + Magnesium Iodide Balanced Equation.
From www.slideserve.com
PPT Balancing Chemical Equations PowerPoint Presentation, free Chlorine + Magnesium Iodide Balanced Equation 1 cl 2 + 1 mgi 2 = 1 mgcl 2 + 1 i 2 for each element, we. Let's balance this equation using the inspection method. This is a bit of a simplification of the reactions but the. Magnesium chloride and iodine with form an equilibrium. Cl2 + mgi2 = mgcl2 + i2 is a single displacement. Chlorine is. Chlorine + Magnesium Iodide Balanced Equation.
From www.numerade.com
SOLVED When the following molecular equation is balanced using the Chlorine + Magnesium Iodide Balanced Equation Let's balance this equation using the inspection method. Explain the role of the law of conservation of mass in a chemical reaction. First, we set all coefficients to 1: Chlorine + magnesium iodide = diiodine + magnesium chloride. Chlorine is in group 7 of the periodic table. Dichlorine + magnesium iodide = magnesium chloride + diiodine. Cl + mgi2 =. Chlorine + Magnesium Iodide Balanced Equation.
From www.numerade.com
SOLVED 14. Chromium + magnesium iodide 15. Chlorine + chromium(III Chlorine + Magnesium Iodide Balanced Equation Chlorine is in group 7 of the periodic table. Magnesium chloride and iodine with form an equilibrium. Chlorine + magnesium iodide = diiodine + magnesium chloride. 1 cl 2 + 1 mgi 2 = 1 mgcl 2 + 1 i 2 for each element, we. Cl2 + mgi2 = mgcl2 + i2 is a single displacement. It is sometimes convenient. Chlorine + Magnesium Iodide Balanced Equation.
From www.numerade.com
SOLVED2_ Write balanced oxidation and reduction halfreaction Chlorine + Magnesium Iodide Balanced Equation Chlorine is in group 7 of the periodic table. 1 cl 2 + 1 mgi 2 = 1 mgcl 2 + 1 i 2 for each element, we. Dichlorine + magnesium iodide = magnesium chloride + diiodine. Explain the role of the law of conservation of mass in a chemical reaction. Chlorine + magnesium iodide = diiodine + magnesium chloride.. Chlorine + Magnesium Iodide Balanced Equation.
From www.chegg.com
Solved Which of the following is the balanced equation for Chlorine + Magnesium Iodide Balanced Equation 1 cl 2 + 1 mgi 2 = 1 mgcl 2 + 1 i 2 for each element, we. In this example the electrons are shown as dots and crosses. Cl + mgi2 = i2 + mgcl2 is a single displacement (substitution). Chlorine is in group 7 of the periodic table. First, we set all coefficients to 1: It is. Chlorine + Magnesium Iodide Balanced Equation.
From www.numerade.com
SOLVED Magnesium metal reacts with chlorine gas, Cl2, to produce Chlorine + Magnesium Iodide Balanced Equation Chlorine + magnesium iodide = diiodine + magnesium chloride. It is sometimes convenient to use fractions instead of integers as intermediate coefficients in the process of balancing a chemical equation. Let's balance this equation using the inspection method. Cl + mgi2 = mgcl2 + i is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one. Chlorine + Magnesium Iodide Balanced Equation.
From www.youtube.com
Equation for MgI2 + H2O (Magnesium iodide + Water) YouTube Chlorine + Magnesium Iodide Balanced Equation Let's balance this equation using the inspection method. Chlorine is in group 7 of the periodic table. Dichlorine + magnesium iodide = magnesium chloride + diiodine. Explain the role of the law of conservation of mass in a chemical reaction. Chlorine + magnesium iodide = diiodine + magnesium chloride. In this example the electrons are shown as dots and crosses.. Chlorine + Magnesium Iodide Balanced Equation.
From slideplayer.com
Writing Chemical Equations. ppt download Chlorine + Magnesium Iodide Balanced Equation First, we set all coefficients to 1: Cl2 + mgi2 = mgcl2 + i2 is a single displacement. 1 cl 2 + 1 mgi 2 = 1 mgcl 2 + 1 i 2 for each element, we. Chlorine is in group 7 of the periodic table. Magnesium chloride and iodine with form an equilibrium. Dichlorine + magnesium iodide = magnesium. Chlorine + Magnesium Iodide Balanced Equation.
From www.toppr.com
8. Write the balanced chemical equation the following and identify the Chlorine + Magnesium Iodide Balanced Equation It is sometimes convenient to use fractions instead of integers as intermediate coefficients in the process of balancing a chemical equation. Magnesium chloride and iodine with form an equilibrium. Cl + mgi2 = mgcl2 + i is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one mole of aqueous. First, we set all coefficients to. Chlorine + Magnesium Iodide Balanced Equation.
From www.youtube.com
How to Balance Mg + I2 = MgI2 (Magnesium + Iodine gas) YouTube Chlorine + Magnesium Iodide Balanced Equation Cl + mgi2 = mgcl2 + i is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one mole of aqueous. Chlorine + magnesium iodide = diiodine + magnesium chloride. Cl2 + mgi2 = mgcl2 + i2 is a single displacement. 1 cl 2 + 1 mgi 2 = 1 mgcl 2 + 1 i 2. Chlorine + Magnesium Iodide Balanced Equation.
From www.numerade.com
SOLVEDMagnesium metal burns in oxygen with an intensely bright white Chlorine + Magnesium Iodide Balanced Equation Cl + mgi2 = mgcl2 + i is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one mole of aqueous. Cl2 + mgi2 = mgcl2 + i2 is a single displacement. Chlorine + magnesium iodide = diiodine + magnesium chloride. Dichlorine + magnesium iodide = magnesium chloride + diiodine. 1 cl 2 + 1 mgi. Chlorine + Magnesium Iodide Balanced Equation.
From signalticket9.pythonanywhere.com
Unbelievable Magnesium Chloride Balanced Equation Maths Formulas For Chlorine + Magnesium Iodide Balanced Equation First, we set all coefficients to 1: Let's balance this equation using the inspection method. This is a bit of a simplification of the reactions but the. Dichlorine + magnesium iodide = magnesium chloride + diiodine. It is sometimes convenient to use fractions instead of integers as intermediate coefficients in the process of balancing a chemical equation. Cl + mgi2. Chlorine + Magnesium Iodide Balanced Equation.
From www.numerade.com
SOLVED Predict the product(s) and write a balanced equation for each Chlorine + Magnesium Iodide Balanced Equation Dichlorine + magnesium iodide = magnesium chloride + diiodine. It is sometimes convenient to use fractions instead of integers as intermediate coefficients in the process of balancing a chemical equation. 1 cl 2 + 1 mgi 2 = 1 mgcl 2 + 1 i 2 for each element, we. Cl + mgi2 = i2 + mgcl2 is a single displacement. Chlorine + Magnesium Iodide Balanced Equation.
From brainly.in
write down balanced equation for heated iron reacts with chlorine Chlorine + Magnesium Iodide Balanced Equation Chlorine + magnesium iodide = diiodine + magnesium chloride. It is sometimes convenient to use fractions instead of integers as intermediate coefficients in the process of balancing a chemical equation. Chlorine is in group 7 of the periodic table. 1 cl 2 + 1 mgi 2 = 1 mgcl 2 + 1 i 2 for each element, we. Explain the. Chlorine + Magnesium Iodide Balanced Equation.
From anastasia-has-harrison.blogspot.com
Aluminum Iodide and Silver I Nitrate Net Ionic Equation Anastasiahas Chlorine + Magnesium Iodide Balanced Equation Explain the role of the law of conservation of mass in a chemical reaction. Let's balance this equation using the inspection method. Chlorine + magnesium iodide = diiodine + magnesium chloride. Cl2 + mgi2 = mgcl2 + i2 is a single displacement. Cl + mgi2 = i2 + mgcl2 is a single displacement (substitution). It is sometimes convenient to use. Chlorine + Magnesium Iodide Balanced Equation.
From www.numerade.com
SOLVED Chlorine with potassium iodide and potassium bromide solutions Chlorine + Magnesium Iodide Balanced Equation Cl + mgi2 = i2 + mgcl2 is a single displacement (substitution). 1 cl 2 + 1 mgi 2 = 1 mgcl 2 + 1 i 2 for each element, we. Explain the role of the law of conservation of mass in a chemical reaction. In this example the electrons are shown as dots and crosses. Balance a chemical equation. Chlorine + Magnesium Iodide Balanced Equation.
From www.toppr.com
quuuuh? What is the need to balance a chemical equation? 7 What happens Chlorine + Magnesium Iodide Balanced Equation Dichlorine + magnesium iodide = magnesium chloride + diiodine. Explain the role of the law of conservation of mass in a chemical reaction. Cl + mgi2 = i2 + mgcl2 is a single displacement (substitution). Let's balance this equation using the inspection method. Chlorine is in group 7 of the periodic table. First, we set all coefficients to 1: Cl. Chlorine + Magnesium Iodide Balanced Equation.
From mavink.com
Magnesium And Hcl Reaction Chlorine + Magnesium Iodide Balanced Equation 1 cl 2 + 1 mgi 2 = 1 mgcl 2 + 1 i 2 for each element, we. Dichlorine + magnesium iodide = magnesium chloride + diiodine. Chlorine + magnesium iodide = diiodine + magnesium chloride. This is a bit of a simplification of the reactions but the. First, we set all coefficients to 1: It is sometimes convenient. Chlorine + Magnesium Iodide Balanced Equation.
From slideplayer.com
Writing Chemical Equations. ppt download Chlorine + Magnesium Iodide Balanced Equation Magnesium chloride and iodine with form an equilibrium. Cl2 + mgi2 = mgcl2 + i2 is a single displacement. 1 cl 2 + 1 mgi 2 = 1 mgcl 2 + 1 i 2 for each element, we. Chlorine + magnesium iodide = diiodine + magnesium chloride. It is sometimes convenient to use fractions instead of integers as intermediate coefficients. Chlorine + Magnesium Iodide Balanced Equation.
From www.numerade.com
SOLVEDPredict the product(s) and write a balanced equation for each of Chlorine + Magnesium Iodide Balanced Equation Let's balance this equation using the inspection method. Balance a chemical equation when given the unbalanced equation. It is sometimes convenient to use fractions instead of integers as intermediate coefficients in the process of balancing a chemical equation. Dichlorine + magnesium iodide = magnesium chloride + diiodine. Chlorine is in group 7 of the periodic table. This is a bit. Chlorine + Magnesium Iodide Balanced Equation.
From ocdrum.com
nickel(ii iodide and potassium carbonate balanced equation) Chlorine + Magnesium Iodide Balanced Equation First, we set all coefficients to 1: Cl + mgi2 = i2 + mgcl2 is a single displacement (substitution). Chlorine is in group 7 of the periodic table. Explain the role of the law of conservation of mass in a chemical reaction. It is sometimes convenient to use fractions instead of integers as intermediate coefficients in the process of balancing. Chlorine + Magnesium Iodide Balanced Equation.
From www.toppr.com
Write the balanced chemical equation the following and identify the of Chlorine + Magnesium Iodide Balanced Equation First, we set all coefficients to 1: Cl + mgi2 = mgcl2 + i is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one mole of aqueous. It is sometimes convenient to use fractions instead of integers as intermediate coefficients in the process of balancing a chemical equation. Chlorine is in group 7 of the. Chlorine + Magnesium Iodide Balanced Equation.
From www.numerade.com
SOLVED When the following molecular equation is balanced using the Chlorine + Magnesium Iodide Balanced Equation Cl + mgi2 = mgcl2 + i is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one mole of aqueous. Let's balance this equation using the inspection method. Explain the role of the law of conservation of mass in a chemical reaction. 1 cl 2 + 1 mgi 2 = 1 mgcl 2 + 1. Chlorine + Magnesium Iodide Balanced Equation.
From www.numerade.com
SOLVED Cl2 + 2KI > 2KCl + I2 In the reaction above, chlorine reacts Chlorine + Magnesium Iodide Balanced Equation This is a bit of a simplification of the reactions but the. 1 cl 2 + 1 mgi 2 = 1 mgcl 2 + 1 i 2 for each element, we. In this example the electrons are shown as dots and crosses. Chlorine + magnesium iodide = diiodine + magnesium chloride. Cl2 + mgi2 = mgcl2 + i2 is a. Chlorine + Magnesium Iodide Balanced Equation.
From socratic.org
How do you write the equation for this reaction Aluminum bromide and Chlorine + Magnesium Iodide Balanced Equation 1 cl 2 + 1 mgi 2 = 1 mgcl 2 + 1 i 2 for each element, we. Cl + mgi2 = mgcl2 + i is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one mole of aqueous. In this example the electrons are shown as dots and crosses. Chlorine is in group 7. Chlorine + Magnesium Iodide Balanced Equation.
From golfcommunications.weebly.com
Aluminum iodide balance chemical equations calculator Chlorine + Magnesium Iodide Balanced Equation This is a bit of a simplification of the reactions but the. Chlorine is in group 7 of the periodic table. Chlorine + magnesium iodide = diiodine + magnesium chloride. First, we set all coefficients to 1: Magnesium chloride and iodine with form an equilibrium. Cl2 + mgi2 = mgcl2 + i2 is a single displacement. Cl + mgi2 =. Chlorine + Magnesium Iodide Balanced Equation.
From www.numerade.com
SOLVED Write balanced equations for the following (i) When chloroform Chlorine + Magnesium Iodide Balanced Equation Cl + mgi2 = mgcl2 + i is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one mole of aqueous. In this example the electrons are shown as dots and crosses. Balance a chemical equation when given the unbalanced equation. Let's balance this equation using the inspection method. It is sometimes convenient to use fractions. Chlorine + Magnesium Iodide Balanced Equation.
From www.toppr.com
Write the balanced chemical equation for the following and identify the Chlorine + Magnesium Iodide Balanced Equation Cl2 + mgi2 = mgcl2 + i2 is a single displacement. Explain the role of the law of conservation of mass in a chemical reaction. Chlorine + magnesium iodide = diiodine + magnesium chloride. Cl + mgi2 = mgcl2 + i is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one mole of aqueous. Cl. Chlorine + Magnesium Iodide Balanced Equation.
From byjus.com
Write word equation and balanced chemical equation for the following Chlorine + Magnesium Iodide Balanced Equation This is a bit of a simplification of the reactions but the. Dichlorine + magnesium iodide = magnesium chloride + diiodine. Chlorine + magnesium iodide = diiodine + magnesium chloride. Cl + mgi2 = mgcl2 + i is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and one mole of aqueous. Explain the role of the. Chlorine + Magnesium Iodide Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for HClO4 + Mg(OH)2 = Mg(ClO4)2 Chlorine + Magnesium Iodide Balanced Equation Chlorine is in group 7 of the periodic table. Chlorine + magnesium iodide = diiodine + magnesium chloride. Explain the role of the law of conservation of mass in a chemical reaction. Dichlorine + magnesium iodide = magnesium chloride + diiodine. Cl + mgi2 = mgcl2 + i is a single displacement (substitution) reaction where two moles of chlorine [cl]. Chlorine + Magnesium Iodide Balanced Equation.
From www.numerade.com
SOLVED Write the balanced chemical equations for the following Chlorine + Magnesium Iodide Balanced Equation Let's balance this equation using the inspection method. Explain the role of the law of conservation of mass in a chemical reaction. 1 cl 2 + 1 mgi 2 = 1 mgcl 2 + 1 i 2 for each element, we. Chlorine + magnesium iodide = diiodine + magnesium chloride. Cl + mgi2 = i2 + mgcl2 is a single. Chlorine + Magnesium Iodide Balanced Equation.
From www.numerade.com
SOLVED1) Please balance the following equations A) Ag2SO4 + BaCI2 Chlorine + Magnesium Iodide Balanced Equation Balance a chemical equation when given the unbalanced equation. First, we set all coefficients to 1: Chlorine + magnesium iodide = diiodine + magnesium chloride. 1 cl 2 + 1 mgi 2 = 1 mgcl 2 + 1 i 2 for each element, we. Explain the role of the law of conservation of mass in a chemical reaction. Cl2 +. Chlorine + Magnesium Iodide Balanced Equation.