Organic Solvent Immiscible . Miscibility most often refers to liquids but. Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates. 47 rows information on the properties of common solvents used in organic chemistry including boiling points, solubility,. Usually one of the solvents is water. Solvent partitioning requires two solvents that are not miscible in each other. A notable exception is that halogenated solvents. Water and low polarity organic solvents. A solvent miscibility table is a valuable tool for scientists and researchers in understanding the compatibility and solubility. The extracting solvent must be immiscible with the solution to be. The other solvent is a liquid that does not dissolve very well in water,.
from www.transtutors.com
Solvent partitioning requires two solvents that are not miscible in each other. A solvent miscibility table is a valuable tool for scientists and researchers in understanding the compatibility and solubility. 47 rows information on the properties of common solvents used in organic chemistry including boiling points, solubility,. Usually one of the solvents is water. The other solvent is a liquid that does not dissolve very well in water,. Miscibility most often refers to liquids but. The extracting solvent must be immiscible with the solution to be. A notable exception is that halogenated solvents. Water and low polarity organic solvents. Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates.
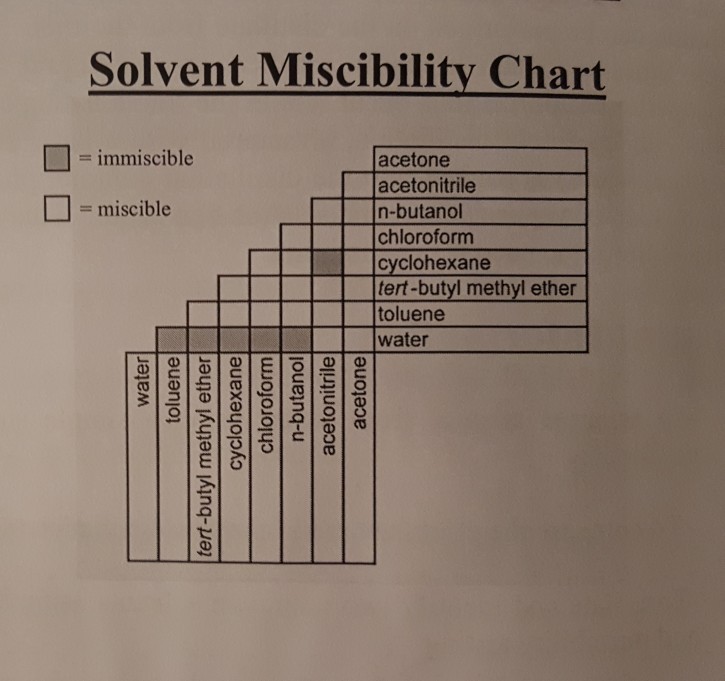
(Get Answer) Solvent Miscibility Chart ?Immiscible ? = Miscible
Organic Solvent Immiscible Solvent partitioning requires two solvents that are not miscible in each other. 47 rows information on the properties of common solvents used in organic chemistry including boiling points, solubility,. A notable exception is that halogenated solvents. Usually one of the solvents is water. A solvent miscibility table is a valuable tool for scientists and researchers in understanding the compatibility and solubility. Solvent partitioning requires two solvents that are not miscible in each other. Water and low polarity organic solvents. Miscibility most often refers to liquids but. Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates. The other solvent is a liquid that does not dissolve very well in water,. The extracting solvent must be immiscible with the solution to be.
From www.masterorganicchemistry.com
All about Solvents NonPolar, Polar Aprotic, and Polar Protic Solvents Organic Solvent Immiscible The extracting solvent must be immiscible with the solution to be. Miscibility most often refers to liquids but. Usually one of the solvents is water. A notable exception is that halogenated solvents. A solvent miscibility table is a valuable tool for scientists and researchers in understanding the compatibility and solubility. The other solvent is a liquid that does not dissolve. Organic Solvent Immiscible.
From www.researchgate.net
EHS assessment results of six organic solvents immiscible with DMSO Organic Solvent Immiscible Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates. Water and low polarity organic solvents. A solvent miscibility table is a valuable tool for scientists and researchers in understanding the compatibility and solubility. The extracting solvent must be immiscible with the solution to be. 47 rows information on the properties of common solvents used in. Organic Solvent Immiscible.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Miscible; immiscible Organic Solvent Immiscible Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates. Miscibility most often refers to liquids but. 47 rows information on the properties of common solvents used in organic chemistry including boiling points, solubility,. The extracting solvent must be immiscible with the solution to be. Usually one of the solvents is water. A notable exception is. Organic Solvent Immiscible.
From www.researchgate.net
Why we use ACETONE in "acetone insolubility" study instead of other Organic Solvent Immiscible A notable exception is that halogenated solvents. Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates. 47 rows information on the properties of common solvents used in organic chemistry including boiling points, solubility,. Solvent partitioning requires two solvents that are not miscible in each other. Usually one of the solvents is water. The other solvent. Organic Solvent Immiscible.
From www.templateroller.com
Common Organic Solvents Table of Properties Yellow Download Printable Organic Solvent Immiscible Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates. A solvent miscibility table is a valuable tool for scientists and researchers in understanding the compatibility and solubility. Water and low polarity organic solvents. 47 rows information on the properties of common solvents used in organic chemistry including boiling points, solubility,. Miscibility most often refers to. Organic Solvent Immiscible.
From www.researchgate.net
Effect of watermiscible and waterimmiscible organic solvents on the Organic Solvent Immiscible The other solvent is a liquid that does not dissolve very well in water,. Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates. Miscibility most often refers to liquids but. A solvent miscibility table is a valuable tool for scientists and researchers in understanding the compatibility and solubility. 47 rows information on the properties of. Organic Solvent Immiscible.
From www.protectoracabrils.org
ciddi anlamda Bozulmamış ekici organic solvent miscibility chart Organic Solvent Immiscible Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates. A solvent miscibility table is a valuable tool for scientists and researchers in understanding the compatibility and solubility. Miscibility most often refers to liquids but. The other solvent is a liquid that does not dissolve very well in water,. Water and low polarity organic solvents. The. Organic Solvent Immiscible.
From www.numerade.com
SOLVED 1. You are mixing benzoic acid with water and an organic Organic Solvent Immiscible Solvent partitioning requires two solvents that are not miscible in each other. The other solvent is a liquid that does not dissolve very well in water,. Miscibility most often refers to liquids but. Water and low polarity organic solvents. Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates. The extracting solvent must be immiscible with. Organic Solvent Immiscible.
From testbook.com
Organic Solvents Learn Meaning, Types, Properties, Lists, Uses Organic Solvent Immiscible The other solvent is a liquid that does not dissolve very well in water,. Water and low polarity organic solvents. Solvent partitioning requires two solvents that are not miscible in each other. The extracting solvent must be immiscible with the solution to be. Usually one of the solvents is water. A notable exception is that halogenated solvents. Miscibility most often. Organic Solvent Immiscible.
From www.gbu-presnenskij.ru
PDF] A REVISION OF THE GUTMANN DONOR NUMBERS OF A SERIES OF, 51 OFF Organic Solvent Immiscible 47 rows information on the properties of common solvents used in organic chemistry including boiling points, solubility,. A notable exception is that halogenated solvents. Solvent partitioning requires two solvents that are not miscible in each other. The extracting solvent must be immiscible with the solution to be. The other solvent is a liquid that does not dissolve very well in. Organic Solvent Immiscible.
From www.researchgate.net
Effect of watermiscible and waterimmiscible organic solvents on the Organic Solvent Immiscible A solvent miscibility table is a valuable tool for scientists and researchers in understanding the compatibility and solubility. The other solvent is a liquid that does not dissolve very well in water,. Miscibility most often refers to liquids but. Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates. Usually one of the solvents is water.. Organic Solvent Immiscible.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Miscible; immiscible Organic Solvent Immiscible Water and low polarity organic solvents. Miscibility most often refers to liquids but. A notable exception is that halogenated solvents. The other solvent is a liquid that does not dissolve very well in water,. Usually one of the solvents is water. The extracting solvent must be immiscible with the solution to be. A solvent miscibility table is a valuable tool. Organic Solvent Immiscible.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Miscible; immiscible Organic Solvent Immiscible Miscibility most often refers to liquids but. Usually one of the solvents is water. The other solvent is a liquid that does not dissolve very well in water,. A notable exception is that halogenated solvents. Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates. Water and low polarity organic solvents. 47 rows information on the. Organic Solvent Immiscible.
From www.researchgate.net
Diagrammatic illustration of liquidliquid extraction (adapted from Organic Solvent Immiscible Water and low polarity organic solvents. Usually one of the solvents is water. Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates. The other solvent is a liquid that does not dissolve very well in water,. The extracting solvent must be immiscible with the solution to be. 47 rows information on the properties of common. Organic Solvent Immiscible.
From www.sigmaaldrich.com
Solvent Miscibility Table Organic Solvent Immiscible 47 rows information on the properties of common solvents used in organic chemistry including boiling points, solubility,. Usually one of the solvents is water. Miscibility most often refers to liquids but. The other solvent is a liquid that does not dissolve very well in water,. A solvent miscibility table is a valuable tool for scientists and researchers in understanding the. Organic Solvent Immiscible.
From www.numerade.com
SOLVED Question 3 When immiscible organic solvent is mixed with water Organic Solvent Immiscible Water and low polarity organic solvents. Solvent partitioning requires two solvents that are not miscible in each other. Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates. A notable exception is that halogenated solvents. The other solvent is a liquid that does not dissolve very well in water,. 47 rows information on the properties of. Organic Solvent Immiscible.
From www.dreamstime.com
Chemical Extraction of Organic Compound from Water Solution To Organic Organic Solvent Immiscible 47 rows information on the properties of common solvents used in organic chemistry including boiling points, solubility,. Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates. The extracting solvent must be immiscible with the solution to be. Water and low polarity organic solvents. A solvent miscibility table is a valuable tool for scientists and researchers. Organic Solvent Immiscible.
From www.chegg.com
Solved Which of the following solvent(s) will form an Organic Solvent Immiscible A notable exception is that halogenated solvents. Solvent partitioning requires two solvents that are not miscible in each other. Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates. 47 rows information on the properties of common solvents used in organic chemistry including boiling points, solubility,. The other solvent is a liquid that does not dissolve. Organic Solvent Immiscible.
From www.transtutors.com
(Get Answer) Solvent Miscibility Chart ?Immiscible ? = Miscible Organic Solvent Immiscible A notable exception is that halogenated solvents. The other solvent is a liquid that does not dissolve very well in water,. 47 rows information on the properties of common solvents used in organic chemistry including boiling points, solubility,. Water and low polarity organic solvents. Miscibility most often refers to liquids but. Solvent partitioning requires two solvents that are not miscible. Organic Solvent Immiscible.
From forum.thefreedictionary.com
immiscible Word of the Day English The Free Dictionary Language Organic Solvent Immiscible Water and low polarity organic solvents. Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates. The other solvent is a liquid that does not dissolve very well in water,. A notable exception is that halogenated solvents. Miscibility most often refers to liquids but. 47 rows information on the properties of common solvents used in organic. Organic Solvent Immiscible.
From www.slideserve.com
PPT Solvent extraction PowerPoint Presentation, free download ID655864 Organic Solvent Immiscible Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates. Usually one of the solvents is water. Miscibility most often refers to liquids but. A notable exception is that halogenated solvents. The extracting solvent must be immiscible with the solution to be. The other solvent is a liquid that does not dissolve very well in water,.. Organic Solvent Immiscible.
From sciencenotes.org
Miscible Definition in Chemistry What Is Miscibility? Organic Solvent Immiscible Water and low polarity organic solvents. The other solvent is a liquid that does not dissolve very well in water,. Usually one of the solvents is water. A solvent miscibility table is a valuable tool for scientists and researchers in understanding the compatibility and solubility. 47 rows information on the properties of common solvents used in organic chemistry including boiling. Organic Solvent Immiscible.
From www.chegg.com
Solved Question 3 When immiscible organic solvent is mixed Organic Solvent Immiscible A solvent miscibility table is a valuable tool for scientists and researchers in understanding the compatibility and solubility. Usually one of the solvents is water. The extracting solvent must be immiscible with the solution to be. A notable exception is that halogenated solvents. Miscibility most often refers to liquids but. Solvent partitioning requires two solvents that are not miscible in. Organic Solvent Immiscible.
From www.chegg.com
Solved A) organic, neither, aqueous, or both B) both, Organic Solvent Immiscible The extracting solvent must be immiscible with the solution to be. Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates. Water and low polarity organic solvents. Usually one of the solvents is water. A solvent miscibility table is a valuable tool for scientists and researchers in understanding the compatibility and solubility. 47 rows information on. Organic Solvent Immiscible.
From www.alamy.com
An illustration of immiscible liquids oil and water mixed together in Organic Solvent Immiscible Miscibility most often refers to liquids but. A solvent miscibility table is a valuable tool for scientists and researchers in understanding the compatibility and solubility. A notable exception is that halogenated solvents. Solvent partitioning requires two solvents that are not miscible in each other. Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates. Usually one. Organic Solvent Immiscible.
From slideplayer.com
Dnyanasadhana College, Thane. Department of Chemistry M. Sc ppt download Organic Solvent Immiscible The extracting solvent must be immiscible with the solution to be. A notable exception is that halogenated solvents. Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates. 47 rows information on the properties of common solvents used in organic chemistry including boiling points, solubility,. Miscibility most often refers to liquids but. Solvent partitioning requires two. Organic Solvent Immiscible.
From stock.adobe.com
Chemical extraction of organic compound from water solution to organic Organic Solvent Immiscible The extracting solvent must be immiscible with the solution to be. Usually one of the solvents is water. Solvent partitioning requires two solvents that are not miscible in each other. A notable exception is that halogenated solvents. Miscibility most often refers to liquids but. Water and low polarity organic solvents. The other solvent is a liquid that does not dissolve. Organic Solvent Immiscible.
From www.researchgate.net
Monitoring the influence of the immiscible organic solvent and Organic Solvent Immiscible Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates. Solvent partitioning requires two solvents that are not miscible in each other. Water and low polarity organic solvents. 47 rows information on the properties of common solvents used in organic chemistry including boiling points, solubility,. A notable exception is that halogenated solvents. A solvent miscibility table. Organic Solvent Immiscible.
From www.researchgate.net
Hi everyone, is there anyone here who did extraction of asccorbyl Organic Solvent Immiscible Miscibility most often refers to liquids but. Water and low polarity organic solvents. The other solvent is a liquid that does not dissolve very well in water,. Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates. A notable exception is that halogenated solvents. A solvent miscibility table is a valuable tool for scientists and researchers. Organic Solvent Immiscible.
From issuu.com
solvent extraction process by Sushanta K Sahu issuu Organic Solvent Immiscible Miscibility most often refers to liquids but. The other solvent is a liquid that does not dissolve very well in water,. Water and low polarity organic solvents. A solvent miscibility table is a valuable tool for scientists and researchers in understanding the compatibility and solubility. A notable exception is that halogenated solvents. The extracting solvent must be immiscible with the. Organic Solvent Immiscible.
From www.numerade.com
VIDEO solution Flow Chart for a Reaction Isolation of Specific Organic Solvent Immiscible Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates. Solvent partitioning requires two solvents that are not miscible in each other. 47 rows information on the properties of common solvents used in organic chemistry including boiling points, solubility,. Usually one of the solvents is water. Water and low polarity organic solvents. Miscibility most often refers. Organic Solvent Immiscible.
From www.chegg.com
Solved Question 3 When immiscible organic solvent is mixed Organic Solvent Immiscible The extracting solvent must be immiscible with the solution to be. Miscibility most often refers to liquids but. 47 rows information on the properties of common solvents used in organic chemistry including boiling points, solubility,. Usually one of the solvents is water. Miscibility defines how substances combine (or mix) to form a homogenous solution without precipitates. The other solvent is. Organic Solvent Immiscible.
From www.chegg.com
Solved 1. Below are structures of few common organic Organic Solvent Immiscible The extracting solvent must be immiscible with the solution to be. Miscibility most often refers to liquids but. A solvent miscibility table is a valuable tool for scientists and researchers in understanding the compatibility and solubility. The other solvent is a liquid that does not dissolve very well in water,. A notable exception is that halogenated solvents. Water and low. Organic Solvent Immiscible.
From www.scribd.com
Solvent Miscibility Chart PDF Tetrahydrofuran Solvent Organic Solvent Immiscible 47 rows information on the properties of common solvents used in organic chemistry including boiling points, solubility,. Solvent partitioning requires two solvents that are not miscible in each other. Usually one of the solvents is water. Miscibility most often refers to liquids but. A notable exception is that halogenated solvents. Miscibility defines how substances combine (or mix) to form a. Organic Solvent Immiscible.
From vdocuments.mx
Hydration of Ions in Organic Solvent and Its Significance in the Gibbs Organic Solvent Immiscible The other solvent is a liquid that does not dissolve very well in water,. Miscibility most often refers to liquids but. Solvent partitioning requires two solvents that are not miscible in each other. Usually one of the solvents is water. The extracting solvent must be immiscible with the solution to be. A notable exception is that halogenated solvents. Miscibility defines. Organic Solvent Immiscible.