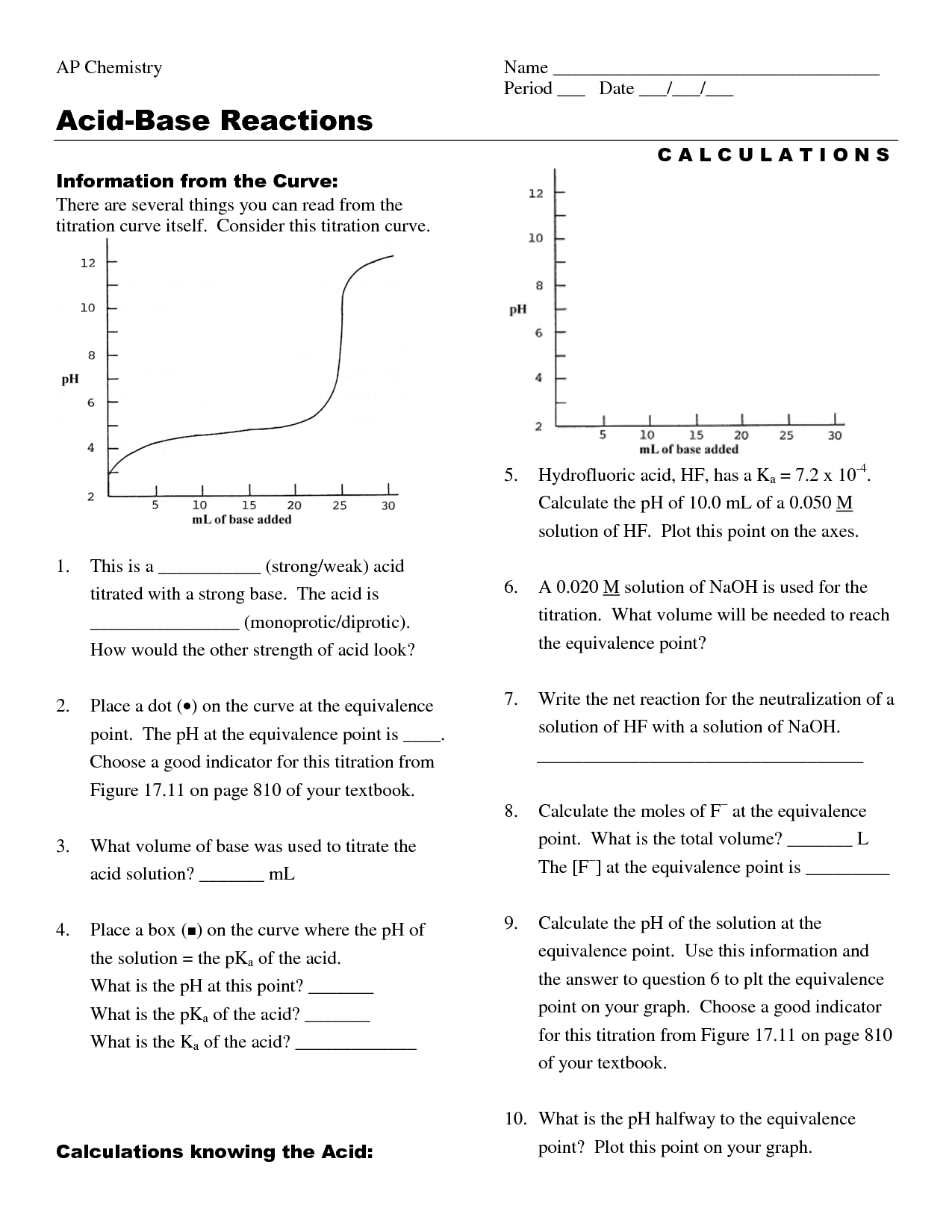
Chemistry Titration Worksheet Answers . If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. What is the concentration of the hcl solution? • a 25 cm3 sample of. Use the information to determine the concentration of the hydrochloric acid. Titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. Titration calculations & answers 1. Give the word equation for the neutralization reaction of an acid and a base. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. If it takes 25 ml. Give your answer to 3 significant figures.
from www.vrogue.co
• a 25 cm3 sample of. Titration calculations & answers 1. Use the information to determine the concentration of the hydrochloric acid. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. If it takes 25 ml. Give the word equation for the neutralization reaction of an acid and a base. What is the concentration of the hcl solution? Titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely.
Titration Calculations Worksheet Gcse Chemistry Beyon vrogue.co
Chemistry Titration Worksheet Answers Titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? What is the concentration of the hcl solution? • a 25 cm3 sample of. Give your answer to 3 significant figures. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Titration calculations & answers 1. Give the word equation for the neutralization reaction of an acid and a base. If it takes 25 ml. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Use the information to determine the concentration of the hydrochloric acid. Titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely.
From www.chemistrylearner.com
Free Printable Acids and Bases Titration Worksheets Chemistry Titration Worksheet Answers Give your answer to 3 significant figures. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. What is the concentration of the hcl solution? Titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. Give the word equation for the neutralization reaction of. Chemistry Titration Worksheet Answers.
From studylib.net
Chemistry 12 Worksheet 46 Anhydrides, Acid Rain and Titrations Chemistry Titration Worksheet Answers Give your answer to 3 significant figures. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? What is the concentration of the hcl solution? Titration is the addition of. Chemistry Titration Worksheet Answers.
From www.scribd.com
GCSE Chemistry Titrations Questions With Answers PDF Chemistry Chemistry Titration Worksheet Answers What is the concentration of the hcl solution? Give the word equation for the neutralization reaction of an acid and a base. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml. Chemistry Titration Worksheet Answers.
From worksheetzonegibson55.z21.web.core.windows.net
Titrations Practice Worksheet Answers Chemistry Titration Worksheet Answers What is the concentration of the hcl solution? Titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. If it takes 25 ml. Use the information to determine the concentration of the hydrochloric acid. • a 25 cm3 sample of. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water. Chemistry Titration Worksheet Answers.
From www.studypool.com
SOLUTION Chemistry titration solved worksheet Studypool Chemistry Titration Worksheet Answers If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Use the information to determine the concentration of the hydrochloric acid. Give your answer to 3 significant figures. Titration is. Chemistry Titration Worksheet Answers.
From worksheets.clipart-library.com
Titration Practice Acid Base Reaction Worksheet with Answer Key Chemistry Titration Worksheet Answers 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. What is the concentration of the hcl solution? • a 25 cm3 sample of. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. If it takes 25 ml. Titration is the. Chemistry Titration Worksheet Answers.
From www.chemistrylearner.com
Free Printable Acids and Bases Titration Worksheets Chemistry Titration Worksheet Answers 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Give your answer to 3 significant figures. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? If it takes 25 ml. Use the information to determine the concentration of. Chemistry Titration Worksheet Answers.
From studyfinder.org
Mastering Acid Base Titration Your Comprehensive Worksheet Answer Key Chemistry Titration Worksheet Answers Use the information to determine the concentration of the hydrochloric acid. What is the concentration of the hcl solution? If it takes 25 ml. • a 25 cm3 sample of. Give your answer to 3 significant figures. Titration calculations & answers 1. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what. Chemistry Titration Worksheet Answers.
From studylib.net
Titration Practice Worksheet Chemistry Titration Worksheet Answers If it takes 25 ml. Titration calculations & answers 1. Give the word equation for the neutralization reaction of an acid and a base. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? • a 25 cm3 sample of. 1) it takes 83 ml of a 0.45 m. Chemistry Titration Worksheet Answers.
From www.docsity.com
Titrations Worksheet 17 with Answers Principles of Chemistry II Chemistry Titration Worksheet Answers Give your answer to 3 significant figures. If it takes 25 ml. Use the information to determine the concentration of the hydrochloric acid. What is the concentration of the hcl solution? Titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. If it takes 54.0 ml of 0.1 m naoh to neutralize 125. Chemistry Titration Worksheet Answers.
From www.vrogue.co
Chemistry Titration Worksheet Answers Chemistryworksheet Com 2019 O Chemistry Titration Worksheet Answers Titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? Titration calculations &. Chemistry Titration Worksheet Answers.
From worksheets.clipart-library.com
Solution and Titration Stoichiometry a Chemistry Worksheet Made Chemistry Titration Worksheet Answers 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. • a 25 cm3 sample of. Use the information to determine the concentration of the hydrochloric acid. Titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. What is the concentration of the hcl. Chemistry Titration Worksheet Answers.
From www.studocu.com
Weak Acid Strong Base Titration Worksheet CHEM 101 Weak Acid and Chemistry Titration Worksheet Answers What is the concentration of the hcl solution? 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Titration calculations & answers 1. Use the information to determine the concentration of the hydrochloric acid. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml. Chemistry Titration Worksheet Answers.
From www.vrogue.co
Titration Calculations Worksheet Gcse Chemistry Beyon vrogue.co Chemistry Titration Worksheet Answers If it takes 25 ml. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is. Chemistry Titration Worksheet Answers.
From www.studypool.com
SOLUTION Chemsheets gcse 1105 titrations 1 ans 93ghs Studypool Chemistry Titration Worksheet Answers Use the information to determine the concentration of the hydrochloric acid. Titration calculations & answers 1. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. What is the concentration of the hcl solution?. Chemistry Titration Worksheet Answers.
From worksheets.clipart-library.com
acid base titration worksheet answer key Worksheets Library Chemistry Titration Worksheet Answers Give the word equation for the neutralization reaction of an acid and a base. Titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. Use the information to determine the concentration of the hydrochloric acid. What is the concentration of the hcl solution? • a 25 cm3 sample of. Titration calculations & answers. Chemistry Titration Worksheet Answers.
From mungfali.com
Titration Worksheet Chemistry Titration Worksheet Answers What is the concentration of the hcl solution? Give the word equation for the neutralization reaction of an acid and a base. • a 25 cm3 sample of. Give your answer to 3 significant figures. If it takes 25 ml. Use the information to determine the concentration of the hydrochloric acid. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is. Chemistry Titration Worksheet Answers.
From mavink.com
Titration Worksheet Chemistry Titration Worksheet Answers • a 25 cm3 sample of. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? Give the word equation for the neutralization reaction of an acid and a base. Titration calculations & answers 1. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235. Chemistry Titration Worksheet Answers.
From printablelibfinance.z13.web.core.windows.net
Titration Practice Problems With Answers Pdf Chemistry Titration Worksheet Answers Titration calculations & answers 1. • a 25 cm3 sample of. Titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. Use the information to determine the concentration of the hydrochloric acid. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. 1) it. Chemistry Titration Worksheet Answers.
From www.chemistryworksheet.com
Chemistry Titration Worksheet Answers Chemistry Titration Worksheet Answers Titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. What is the concentration of the hcl solution? Use the information to determine the concentration of the hydrochloric acid. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? If it takes. Chemistry Titration Worksheet Answers.
From www.edplace.com
Understand Titration Worksheet EdPlace Chemistry Titration Worksheet Answers A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Use the information to determine the concentration of the hydrochloric acid. If it takes 25 ml. Titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. Give the word equation for the neutralization reaction. Chemistry Titration Worksheet Answers.
From worksheetzonegibson55.z21.web.core.windows.net
Titrations Practice Worksheet Answers Chemistry Titration Worksheet Answers What is the concentration of the hcl solution? Use the information to determine the concentration of the hydrochloric acid. Titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. • a 25 cm3 sample of. Give your answer to 3 significant figures. If it takes 25 ml. Give the word equation for the. Chemistry Titration Worksheet Answers.
From www.tes.com
Edexcel IGCSE Chemistry Worksheet 22 Titrations Teaching Resources Chemistry Titration Worksheet Answers Give your answer to 3 significant figures. Give the word equation for the neutralization reaction of an acid and a base. What is the concentration of the hcl solution? • a 25 cm3 sample of. Use the information to determine the concentration of the hydrochloric acid. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of. Chemistry Titration Worksheet Answers.
From philo-khosravani.blogspot.com
Acid Base Titration Worksheet Answers philokhosravani Chemistry Titration Worksheet Answers If it takes 25 ml. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? What is the concentration of the hcl solution? Give your answer to 3 significant figures. Use the information to determine the concentration of the hydrochloric acid. Give the word equation for the neutralization reaction. Chemistry Titration Worksheet Answers.
From education-com-worksheets.blogspot.com
Acid Base Titration Worksheet Answers Education Com Worksheets Chemistry Titration Worksheet Answers Titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? Use the information to determine the concentration of the hydrochloric acid. • a 25 cm3 sample of. What is the concentration of. Chemistry Titration Worksheet Answers.
From myans.bhantedhammika.net
Acids And Bases Titration Worksheet Answer Key Chemistry Titration Worksheet Answers Titration calculations & answers 1. If it takes 25 ml. Give the word equation for the neutralization reaction of an acid and a base. • a 25 cm3 sample of. What is the concentration of the hcl solution? Titration is the addition of a standard solution of precisely known concentration (the titrant) to a precisely. 1) it takes 83 ml. Chemistry Titration Worksheet Answers.
From dorkiestenews.blogspot.com
Acid Base Titration Worksheet Answers Acid Base Titration Worksheet Chemistry Titration Worksheet Answers Give your answer to 3 significant figures. • a 25 cm3 sample of. Give the word equation for the neutralization reaction of an acid and a base. Use the information to determine the concentration of the hydrochloric acid. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. A 1.0000 gram. Chemistry Titration Worksheet Answers.
From www.studocu.com
Acidbases Titrations worksheet Acid Bases Titrations Chapter What Chemistry Titration Worksheet Answers 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. • a 25 cm3 sample of. Give the word equation for the neutralization reaction of an acid and a base. If it takes 25 ml. Titration calculations & answers 1. If it takes 54.0 ml of 0.1 m naoh to neutralize. Chemistry Titration Worksheet Answers.
From quizzlistjonas.z19.web.core.windows.net
Titration Worksheet Answer Key Chemistry Titration Worksheet Answers • a 25 cm3 sample of. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? Titration calculations & answers 1. What is the concentration of the hcl solution? Give your answer to 3 significant figures. If it takes 25 ml. Use the information to determine the concentration of. Chemistry Titration Worksheet Answers.
From www.studocu.com
Worksheet on Chapter 20 Titration Essentials of Chemistry Summer Chemistry Titration Worksheet Answers What is the concentration of the hcl solution? Use the information to determine the concentration of the hydrochloric acid. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? If it takes 25 ml. Give your answer to 3 significant figures. 1) it takes 83 ml of a 0.45. Chemistry Titration Worksheet Answers.
From cowchem.weebly.com
Redox Titrations MS MCLARTY'S CLASSES Chemistry Titration Worksheet Answers Give your answer to 3 significant figures. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? Give the word equation for the neutralization reaction of an acid and a. Chemistry Titration Worksheet Answers.
From rocketsheets.co.uk
Titrations Home Learning Worksheet GCSE rocketsheets.co.uk Chemistry Titration Worksheet Answers Titration calculations & answers 1. Use the information to determine the concentration of the hydrochloric acid. If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? Give the word equation for the neutralization reaction of an acid and a base. • a 25 cm3 sample of. 1) it takes. Chemistry Titration Worksheet Answers.
From rocketsheets.co.uk
Titrations Home Learning Worksheet GCSE rocketsheets.co.uk Chemistry Titration Worksheet Answers Give your answer to 3 significant figures. Titration calculations & answers 1. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. • a 25 cm3 sample of. Titration is the addition. Chemistry Titration Worksheet Answers.
From www.scribd.com
gizmo titration worksheet Titration Chemistry Chemistry Titration Worksheet Answers If it takes 54.0 ml of 0.1 m naoh to neutralize 125 ml of an hcl solution, what is the [hcl]? Give your answer to 3 significant figures. • a 25 cm3 sample of. Use the information to determine the concentration of the hydrochloric acid. Titration is the addition of a standard solution of precisely known concentration (the titrant) to. Chemistry Titration Worksheet Answers.
From www.chemistryworksheet.com
Chemistry Titration Worksheet Answers Chemistry Titration Worksheet Answers A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Use the information to determine the concentration of the hydrochloric acid. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Give the word equation for the neutralization reaction of an acid. Chemistry Titration Worksheet Answers.