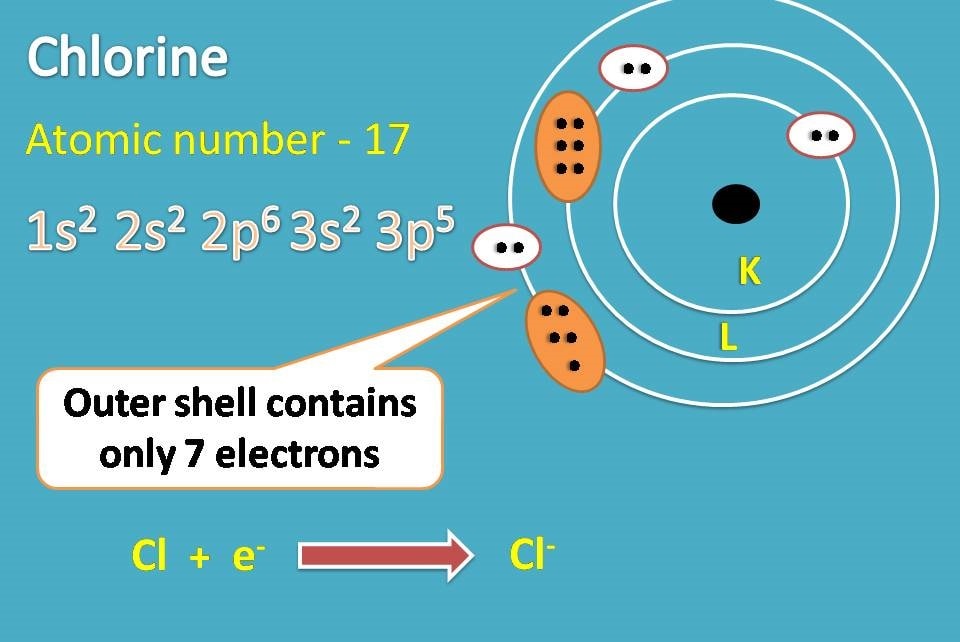
Chlorine Ion Configuration . #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine ion (. We add electrons to fill the outermost orbital that is occupied, and. Learn about the electron configuration of chlorine ([ne]3s2 3p5) and its chemical reactions with water, oxygen, hydrogen, and metals. The electron configuration of a chlorine atom (#cl#) is as follows: See the table with atomic number, element name, shorthand, full configuration. The electronic configuration of anions is assigned by adding electrons according to aufbau principle. Learn how to write electron configuration for all elements using shorthand and full notation. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges.
from egpat.com
Learn about the electron configuration of chlorine ([ne]3s2 3p5) and its chemical reactions with water, oxygen, hydrogen, and metals. We add electrons to fill the outermost orbital that is occupied, and. See the table with atomic number, element name, shorthand, full configuration. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine ion (. The electron configuration of a chlorine atom (#cl#) is as follows: The electronic configuration of anions is assigned by adding electrons according to aufbau principle. Learn how to write electron configuration for all elements using shorthand and full notation.
Lewis dot structure How to write?
Chlorine Ion Configuration Learn about the electron configuration of chlorine ([ne]3s2 3p5) and its chemical reactions with water, oxygen, hydrogen, and metals. We add electrons to fill the outermost orbital that is occupied, and. Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Learn how to write electron configuration for all elements using shorthand and full notation. See the table with atomic number, element name, shorthand, full configuration. The electron configuration of a chlorine atom (#cl#) is as follows: #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine ion (. Learn about the electron configuration of chlorine ([ne]3s2 3p5) and its chemical reactions with water, oxygen, hydrogen, and metals. The electronic configuration of anions is assigned by adding electrons according to aufbau principle.
From www.doubtnut.com
(a) Give the schematic atomic structures of chlorine atom and chloride Chlorine Ion Configuration #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine ion (. The electron configuration of a chlorine atom (#cl#) is as follows: Learn how to write electron configuration for all elements using shorthand and full notation. Learn about the electron configuration of chlorine ([ne]3s2 3p5) and its chemical reactions with water, oxygen, hydrogen, and metals. In order to write the chlorine. Chlorine Ion Configuration.
From www.youtube.com
Cl Electron Configuration (Chloride Ion) YouTube Chlorine Ion Configuration The electronic configuration of anions is assigned by adding electrons according to aufbau principle. The electron configuration of a chlorine atom (#cl#) is as follows: We add electrons to fill the outermost orbital that is occupied, and. #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine ion (. In order to write the chlorine electron configuration we first need to know. Chlorine Ion Configuration.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille Chlorine Ion Configuration Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. The electron configuration of a chlorine atom (#cl#) is as follows: The electronic configuration of anions is assigned by adding electrons according to aufbau principle. In order to write the chlorine electron configuration we first need to know the. Chlorine Ion Configuration.
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition and electron Chlorine Ion Configuration We add electrons to fill the outermost orbital that is occupied, and. See the table with atomic number, element name, shorthand, full configuration. The electronic configuration of anions is assigned by adding electrons according to aufbau principle. The electron configuration of a chlorine atom (#cl#) is as follows: Learn about the electron configuration of chlorine ([ne]3s2 3p5) and its chemical. Chlorine Ion Configuration.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Chlorine Ion Configuration We add electrons to fill the outermost orbital that is occupied, and. Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. See the table with atomic number, element name, shorthand, full configuration. The electron configuration of a chlorine atom (#cl#) is as follows: Learn about the electron configuration. Chlorine Ion Configuration.
From www.slideserve.com
PPT Electron Energy Levels and Configurations PowerPoint Presentation Chlorine Ion Configuration The electron configuration of a chlorine atom (#cl#) is as follows: The electronic configuration of anions is assigned by adding electrons according to aufbau principle. #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine ion (. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons).. Chlorine Ion Configuration.
From www.expii.com
Ions — Definition & Overview Expii Chlorine Ion Configuration In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). See the table with atomic number, element name, shorthand, full configuration. #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine ion (. Learn how to write electron configuration for all elements using shorthand and full notation.. Chlorine Ion Configuration.
From www.youtube.com
Cl(Chloride ion) and Cl(Chlorine) Electron Configuration,Orbital Chlorine Ion Configuration In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). The electron configuration of a chlorine atom (#cl#) is as follows: Learn about the electron configuration of chlorine ([ne]3s2 3p5) and its chemical reactions with water, oxygen, hydrogen, and metals. The electronic configuration of anions. Chlorine Ion Configuration.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Ion Configuration The electronic configuration of anions is assigned by adding electrons according to aufbau principle. Learn about the electron configuration of chlorine ([ne]3s2 3p5) and its chemical reactions with water, oxygen, hydrogen, and metals. #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine ion (. We add electrons to fill the outermost orbital that is occupied, and. In order to write the. Chlorine Ion Configuration.
From egpat.com
Lewis dot structure How to write? Chlorine Ion Configuration In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Learn how to write electron configuration for all elements using shorthand and full notation. Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges.. Chlorine Ion Configuration.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Ion Configuration Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. The electronic configuration of anions is assigned by adding electrons according to aufbau principle. Learn about the electron configuration of chlorine ([ne]3s2 3p5) and its chemical reactions with water, oxygen, hydrogen, and metals. We add electrons to fill the. Chlorine Ion Configuration.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Chlorine Ion Configuration Learn about the electron configuration of chlorine ([ne]3s2 3p5) and its chemical reactions with water, oxygen, hydrogen, and metals. The electron configuration of a chlorine atom (#cl#) is as follows: In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). #1s^2 2s^2 2p^6 3s^2 3p^5#. Chlorine Ion Configuration.
From www.shutterstock.com
Atom Chlorine This Diagram Shows Electron Stock Vector 328668782 Chlorine Ion Configuration The electronic configuration of anions is assigned by adding electrons according to aufbau principle. Learn how to write electron configuration for all elements using shorthand and full notation. The electron configuration of a chlorine atom (#cl#) is as follows: Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges.. Chlorine Ion Configuration.
From ar.inspiredpencil.com
Electron Configuration For Chlorine Chlorine Ion Configuration The electron configuration of a chlorine atom (#cl#) is as follows: Learn about the electron configuration of chlorine ([ne]3s2 3p5) and its chemical reactions with water, oxygen, hydrogen, and metals. Learn how to write electron configuration for all elements using shorthand and full notation. The electronic configuration of anions is assigned by adding electrons according to aufbau principle. We add. Chlorine Ion Configuration.
From www.researchgate.net
Typical configuration of Cl(H 2 O) 60 cluster at 210 K. Chlorine ion Chlorine Ion Configuration The electronic configuration of anions is assigned by adding electrons according to aufbau principle. #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine ion (. Learn how to write electron configuration for all elements using shorthand and full notation. Learn about the electron configuration of chlorine ([ne]3s2 3p5) and its chemical reactions with water, oxygen, hydrogen, and metals. In order to. Chlorine Ion Configuration.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Ion Configuration Learn about the electron configuration of chlorine ([ne]3s2 3p5) and its chemical reactions with water, oxygen, hydrogen, and metals. The electronic configuration of anions is assigned by adding electrons according to aufbau principle. The electron configuration of a chlorine atom (#cl#) is as follows: #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine ion (. Learn how to write electron configuration. Chlorine Ion Configuration.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Ion Configuration The electron configuration of a chlorine atom (#cl#) is as follows: We add electrons to fill the outermost orbital that is occupied, and. Learn about the electron configuration of chlorine ([ne]3s2 3p5) and its chemical reactions with water, oxygen, hydrogen, and metals. #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine ion (. The electronic configuration of anions is assigned by. Chlorine Ion Configuration.
From www.shutterstock.com
3.722 Atom structure of chlorine Görseli, Stok Fotoğraflar ve Vektörler Chlorine Ion Configuration Learn how to write electron configuration for all elements using shorthand and full notation. Learn about the electron configuration of chlorine ([ne]3s2 3p5) and its chemical reactions with water, oxygen, hydrogen, and metals. Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. In order to write the chlorine. Chlorine Ion Configuration.
From studiousguy.com
Chlorine (Cl) Properties & Uses StudiousGuy Chlorine Ion Configuration The electronic configuration of anions is assigned by adding electrons according to aufbau principle. The electron configuration of a chlorine atom (#cl#) is as follows: Learn about the electron configuration of chlorine ([ne]3s2 3p5) and its chemical reactions with water, oxygen, hydrogen, and metals. #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine ion (. We add electrons to fill the. Chlorine Ion Configuration.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions Chlorine Ion Configuration In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine ion (. See the table with atomic number, element name, shorthand, full configuration. Learn how to determine the electron configuration of cations and anions, and how to. Chlorine Ion Configuration.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Ion Configuration #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine ion (. The electronic configuration of anions is assigned by adding electrons according to aufbau principle. We add electrons to fill the outermost orbital that is occupied, and. See the table with atomic number, element name, shorthand, full configuration. Learn how to write electron configuration for all elements using shorthand and full. Chlorine Ion Configuration.
From sciencenotes.org
Chlorine Facts Chlorine Ion Configuration In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. See the table with atomic number, element name, shorthand, full configuration. Learn how to. Chlorine Ion Configuration.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Ion Configuration We add electrons to fill the outermost orbital that is occupied, and. Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. The electronic configuration of anions is assigned by adding electrons according to aufbau principle. See the table with atomic number, element name, shorthand, full configuration. Learn about. Chlorine Ion Configuration.
From www.embibe.com
Draw the atomic structure of the Chlorine atom and chlorine ion Chlorine Ion Configuration The electron configuration of a chlorine atom (#cl#) is as follows: In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Learn about the electron configuration of chlorine ([ne]3s2 3p5) and its chemical reactions with water, oxygen, hydrogen, and metals. The electronic configuration of anions. Chlorine Ion Configuration.
From www.bartleby.com
B. A chlorine atom (Cl) a negatively charged chloride ion (Cl − Chlorine Ion Configuration See the table with atomic number, element name, shorthand, full configuration. Learn how to write electron configuration for all elements using shorthand and full notation. Learn about the electron configuration of chlorine ([ne]3s2 3p5) and its chemical reactions with water, oxygen, hydrogen, and metals. In order to write the chlorine electron configuration we first need to know the number of. Chlorine Ion Configuration.
From favpng.com
Atom Bohr Model Electron Configuration Chlorine, PNG, 1000x1000px, Atom Chlorine Ion Configuration In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Learn about the electron configuration of chlorine ([ne]3s2 3p5) and its chemical reactions with water, oxygen, hydrogen, and metals. #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine ion (. The electron configuration of a chlorine. Chlorine Ion Configuration.
From brainly.in
Draw the atomic structure of a chlorine ion Brainly.in Chlorine Ion Configuration See the table with atomic number, element name, shorthand, full configuration. We add electrons to fill the outermost orbital that is occupied, and. #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine ion (. The electron configuration of a chlorine atom (#cl#) is as follows: In order to write the chlorine electron configuration we first need to know the number of. Chlorine Ion Configuration.
From pixels.com
Chlorine Electron Configuration Photograph by Chlorine Ion Configuration Learn how to write electron configuration for all elements using shorthand and full notation. The electronic configuration of anions is assigned by adding electrons according to aufbau principle. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). The electron configuration of a chlorine atom. Chlorine Ion Configuration.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Ion Configuration We add electrons to fill the outermost orbital that is occupied, and. Learn how to write electron configuration for all elements using shorthand and full notation. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Learn about the electron configuration of chlorine ([ne]3s2 3p5). Chlorine Ion Configuration.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Chlorine Ion Configuration Learn about the electron configuration of chlorine ([ne]3s2 3p5) and its chemical reactions with water, oxygen, hydrogen, and metals. #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine ion (. We add electrons to fill the outermost orbital that is occupied, and. See the table with atomic number, element name, shorthand, full configuration. The electronic configuration of anions is assigned by. Chlorine Ion Configuration.
From www.chemistrylearner.com
Chlorine Facts, Symbol, Discovery, Properties, Uses Chlorine Ion Configuration See the table with atomic number, element name, shorthand, full configuration. The electronic configuration of anions is assigned by adding electrons according to aufbau principle. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Learn how to determine the electron configuration of cations and. Chlorine Ion Configuration.
From www.youtube.com
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube Chlorine Ion Configuration #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine ion (. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). The electronic configuration of anions is assigned by adding electrons according to aufbau principle. The electron configuration of a chlorine atom (#cl#) is as follows:. Chlorine Ion Configuration.
From kunduz.com
[ANSWERED] The electron dot diagram for a neutral at... Physical Chlorine Ion Configuration Learn about the electron configuration of chlorine ([ne]3s2 3p5) and its chemical reactions with water, oxygen, hydrogen, and metals. The electronic configuration of anions is assigned by adding electrons according to aufbau principle. See the table with atomic number, element name, shorthand, full configuration. #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine ion (. Learn how to determine the electron. Chlorine Ion Configuration.
From www.askiitians.com
Chlorine Study Material for IIT JEE askIITians Chlorine Ion Configuration We add electrons to fill the outermost orbital that is occupied, and. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). See the table with atomic number, element name, shorthand, full configuration. #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine ion (. The electron. Chlorine Ion Configuration.
From www.youtube.com
How to find Protons & Electrons for the Chloride ion (Cl) YouTube Chlorine Ion Configuration The electron configuration of a chlorine atom (#cl#) is as follows: Learn how to determine the electron configuration of cations and anions, and how to use magnetic properties to justify their charges. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Learn how to. Chlorine Ion Configuration.