What Is A Medical Device Registry . Clinical data registries are also sometimes. What is an outcome registry? The medical devices and outcomes registry. Outcome registries are organised systems that use observational methods to collect routine, uniform data on. A clinical data registry is an interactive database that collects, organizes, and displays healthcare information. This was an existing nhs england collection under the surgical devices. Medical devices need to be registered with mhra after they have been certified by an uk approved body, an eu notified body, or where they have. According to the medical devices regulations 2002 (si 2002 no 618, as amended) (uk mdr 2002), a medical device is described. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as.
from www.evnia.dk
A clinical data registry is an interactive database that collects, organizes, and displays healthcare information. According to the medical devices regulations 2002 (si 2002 no 618, as amended) (uk mdr 2002), a medical device is described. Outcome registries are organised systems that use observational methods to collect routine, uniform data on. Medical devices need to be registered with mhra after they have been certified by an uk approved body, an eu notified body, or where they have. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. The medical devices and outcomes registry. Clinical data registries are also sometimes. This was an existing nhs england collection under the surgical devices. What is an outcome registry?
Registries Evnia
What Is A Medical Device Registry A clinical data registry is an interactive database that collects, organizes, and displays healthcare information. What is an outcome registry? According to the medical devices regulations 2002 (si 2002 no 618, as amended) (uk mdr 2002), a medical device is described. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. This was an existing nhs england collection under the surgical devices. Medical devices need to be registered with mhra after they have been certified by an uk approved body, an eu notified body, or where they have. A clinical data registry is an interactive database that collects, organizes, and displays healthcare information. The medical devices and outcomes registry. Clinical data registries are also sometimes. Outcome registries are organised systems that use observational methods to collect routine, uniform data on.
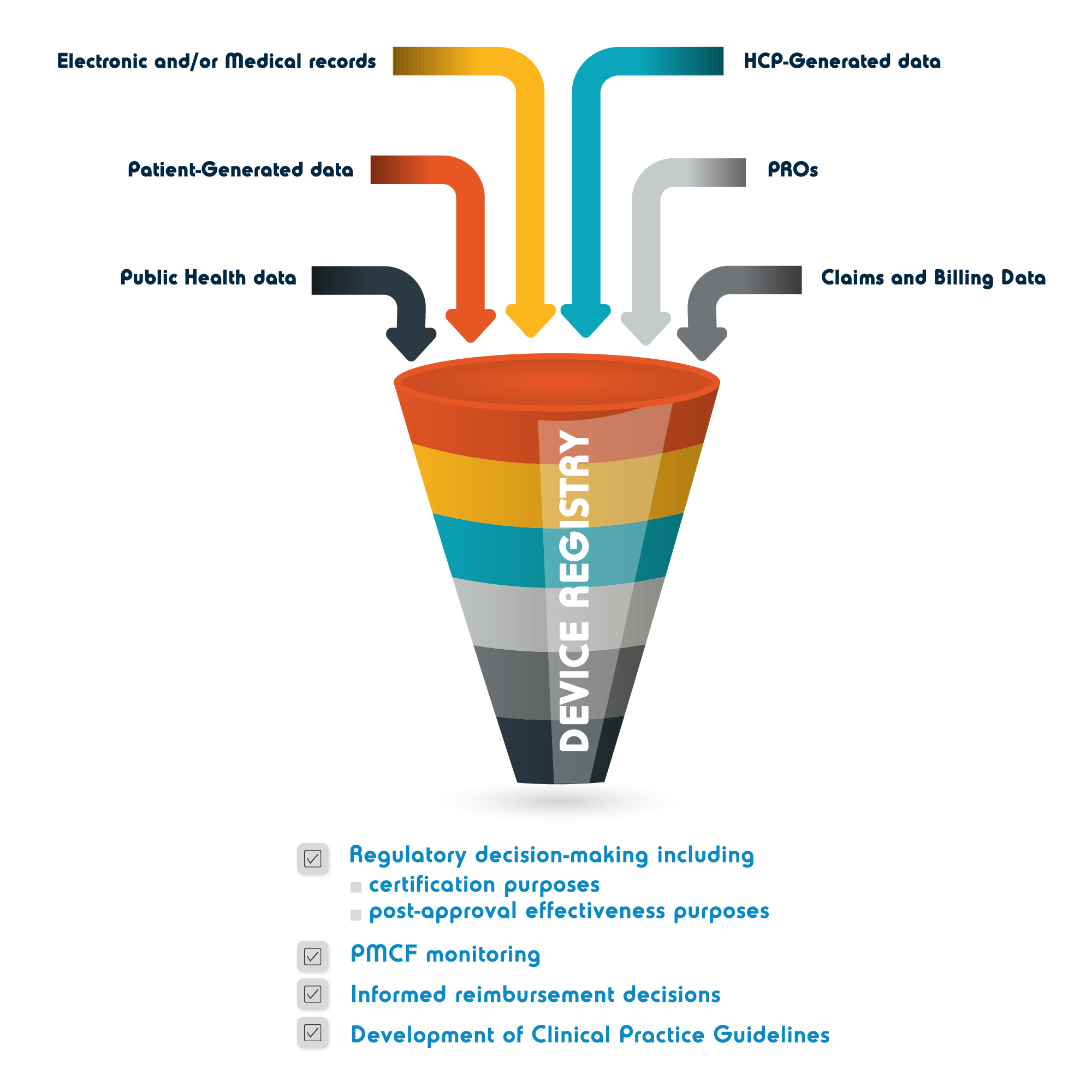
From medicaldeviceregistry.com
What is the Bernstein test and how does it work? Medical Device Registry What Is A Medical Device Registry Clinical data registries are also sometimes. According to the medical devices regulations 2002 (si 2002 no 618, as amended) (uk mdr 2002), a medical device is described. Medical devices need to be registered with mhra after they have been certified by an uk approved body, an eu notified body, or where they have. This was an existing nhs england collection. What Is A Medical Device Registry.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements What Is A Medical Device Registry What is an outcome registry? A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. This was an existing nhs england collection under the surgical devices. Outcome registries are organised systems that use observational methods to collect routine, uniform data on. Medical devices need. What Is A Medical Device Registry.
From www.facebook.com
STEPS FOR MEDICAL DEVICE REGISTRATION Here are the steps if you still What Is A Medical Device Registry Clinical data registries are also sometimes. This was an existing nhs england collection under the surgical devices. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. What is an outcome registry? The medical devices and outcomes registry. Outcome registries are organised systems that. What Is A Medical Device Registry.
From arogyalegal.com
Frequently Asked Questions on new registration requirement for medical What Is A Medical Device Registry Outcome registries are organised systems that use observational methods to collect routine, uniform data on. What is an outcome registry? According to the medical devices regulations 2002 (si 2002 no 618, as amended) (uk mdr 2002), a medical device is described. This was an existing nhs england collection under the surgical devices. A medical device is any instrument, apparatus, appliance,. What Is A Medical Device Registry.
From www.access.preprod.fda.gov
Register a New Medical Device Facility StepbyStep Instructions What Is A Medical Device Registry The medical devices and outcomes registry. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. What is an outcome registry? Outcome registries are organised systems that use observational methods to collect routine, uniform data on. A clinical data registry is an interactive database. What Is A Medical Device Registry.
From www.meddevicecorp.com
US FDA Registration Service of Food, Drugs, Medical Devices What Is A Medical Device Registry The medical devices and outcomes registry. According to the medical devices regulations 2002 (si 2002 no 618, as amended) (uk mdr 2002), a medical device is described. What is an outcome registry? Outcome registries are organised systems that use observational methods to collect routine, uniform data on. Medical devices need to be registered with mhra after they have been certified. What Is A Medical Device Registry.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero What Is A Medical Device Registry The medical devices and outcomes registry. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. Outcome registries are organised systems that use observational methods to collect routine, uniform data on. Medical devices need to be registered with mhra after they have been certified. What Is A Medical Device Registry.
From www.regdesk.co
MHRA Guidance on Registration of Medical Devices RegDesk What Is A Medical Device Registry The medical devices and outcomes registry. According to the medical devices regulations 2002 (si 2002 no 618, as amended) (uk mdr 2002), a medical device is described. Outcome registries are organised systems that use observational methods to collect routine, uniform data on. This was an existing nhs england collection under the surgical devices. A medical device is any instrument, apparatus,. What Is A Medical Device Registry.
From fdapals.com
FDA Medical Device Registration FDApals What Is A Medical Device Registry What is an outcome registry? Outcome registries are organised systems that use observational methods to collect routine, uniform data on. According to the medical devices regulations 2002 (si 2002 no 618, as amended) (uk mdr 2002), a medical device is described. A clinical data registry is an interactive database that collects, organizes, and displays healthcare information. Medical devices need to. What Is A Medical Device Registry.
From medtechintelligence.com
Column Compliance Date Approaching for FDA Unique Device Identifiers What Is A Medical Device Registry A clinical data registry is an interactive database that collects, organizes, and displays healthcare information. Medical devices need to be registered with mhra after they have been certified by an uk approved body, an eu notified body, or where they have. What is an outcome registry? A medical device is any instrument, apparatus, appliance, software, material, or other article, which. What Is A Medical Device Registry.
From www.evnia.dk
Registries Evnia What Is A Medical Device Registry According to the medical devices regulations 2002 (si 2002 no 618, as amended) (uk mdr 2002), a medical device is described. A clinical data registry is an interactive database that collects, organizes, and displays healthcare information. What is an outcome registry? The medical devices and outcomes registry. Clinical data registries are also sometimes. A medical device is any instrument, apparatus,. What Is A Medical Device Registry.
From www.companisto.com
The Patient Registry Is an Important Asset for EBS What Is A Medical Device Registry Medical devices need to be registered with mhra after they have been certified by an uk approved body, an eu notified body, or where they have. Clinical data registries are also sometimes. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. A clinical. What Is A Medical Device Registry.
From www.amazon.co.jp
Amazon Medical Device Register 1997 International Edition (Annual What Is A Medical Device Registry A clinical data registry is an interactive database that collects, organizes, and displays healthcare information. The medical devices and outcomes registry. What is an outcome registry? Medical devices need to be registered with mhra after they have been certified by an uk approved body, an eu notified body, or where they have. Outcome registries are organised systems that use observational. What Is A Medical Device Registry.
From www.orielstat.com
Class 1 Medical Device Requirements Oriel STAT A MATRIX What Is A Medical Device Registry A clinical data registry is an interactive database that collects, organizes, and displays healthcare information. What is an outcome registry? Clinical data registries are also sometimes. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. Outcome registries are organised systems that use observational. What Is A Medical Device Registry.
From angelanjohnson.com
Medical Devices Angela N Johnson What Is A Medical Device Registry This was an existing nhs england collection under the surgical devices. Medical devices need to be registered with mhra after they have been certified by an uk approved body, an eu notified body, or where they have. A clinical data registry is an interactive database that collects, organizes, and displays healthcare information. What is an outcome registry? According to the. What Is A Medical Device Registry.
From www.access.fda.gov
Register a New Medical Device Facility StepbyStep Instructions What Is A Medical Device Registry A clinical data registry is an interactive database that collects, organizes, and displays healthcare information. What is an outcome registry? According to the medical devices regulations 2002 (si 2002 no 618, as amended) (uk mdr 2002), a medical device is described. The medical devices and outcomes registry. Medical devices need to be registered with mhra after they have been certified. What Is A Medical Device Registry.
From transcustoms.com
Medical Device NMPA Registration Procedrue Flow Chart What Is A Medical Device Registry Medical devices need to be registered with mhra after they have been certified by an uk approved body, an eu notified body, or where they have. Outcome registries are organised systems that use observational methods to collect routine, uniform data on. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use. What Is A Medical Device Registry.
From www.pewtrusts.org
Medical Device Registries The Pew Charitable Trusts What Is A Medical Device Registry This was an existing nhs england collection under the surgical devices. Clinical data registries are also sometimes. The medical devices and outcomes registry. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. What is an outcome registry? According to the medical devices regulations. What Is A Medical Device Registry.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Medical Device Classification What Is A Medical Device Registry The medical devices and outcomes registry. This was an existing nhs england collection under the surgical devices. According to the medical devices regulations 2002 (si 2002 no 618, as amended) (uk mdr 2002), a medical device is described. A clinical data registry is an interactive database that collects, organizes, and displays healthcare information. Outcome registries are organised systems that use. What Is A Medical Device Registry.
From www.slideserve.com
PPT How do you register your medical device with CDSCO? Regulatory What Is A Medical Device Registry The medical devices and outcomes registry. A clinical data registry is an interactive database that collects, organizes, and displays healthcare information. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. According to the medical devices regulations 2002 (si 2002 no 618, as amended). What Is A Medical Device Registry.
From www.researchgate.net
(PDF) Design, Implementation and Management of an International Medical What Is A Medical Device Registry A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. A clinical data registry is an interactive database that collects, organizes, and displays healthcare information. According to the medical devices regulations 2002 (si 2002 no 618, as amended) (uk mdr 2002), a medical device. What Is A Medical Device Registry.
From www.rizmona.com
Medical Device Registration in the UAE Riz & Mona What Is A Medical Device Registry What is an outcome registry? A clinical data registry is an interactive database that collects, organizes, and displays healthcare information. Medical devices need to be registered with mhra after they have been certified by an uk approved body, an eu notified body, or where they have. According to the medical devices regulations 2002 (si 2002 no 618, as amended) (uk. What Is A Medical Device Registry.
From www.arbormetrix.com
An Intro to Big Data Analytics in Healthcare ArborMetrix What Is A Medical Device Registry A clinical data registry is an interactive database that collects, organizes, and displays healthcare information. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. The medical devices and outcomes registry. Clinical data registries are also sometimes. According to the medical devices regulations 2002. What Is A Medical Device Registry.
From www.greenlight.guru
5 Tips for Medical Device Registration across Global Markets What Is A Medical Device Registry The medical devices and outcomes registry. According to the medical devices regulations 2002 (si 2002 no 618, as amended) (uk mdr 2002), a medical device is described. This was an existing nhs england collection under the surgical devices. A clinical data registry is an interactive database that collects, organizes, and displays healthcare information. A medical device is any instrument, apparatus,. What Is A Medical Device Registry.
From www.freyrsolutions.com
Medical Device Registration in India for Foreign Manufactures Freyr What Is A Medical Device Registry Medical devices need to be registered with mhra after they have been certified by an uk approved body, an eu notified body, or where they have. The medical devices and outcomes registry. What is an outcome registry? A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical. What Is A Medical Device Registry.
From www.amazon.co.uk
Medical Device Register (Medical Device Register, Domestic Edition What Is A Medical Device Registry The medical devices and outcomes registry. Medical devices need to be registered with mhra after they have been certified by an uk approved body, an eu notified body, or where they have. A clinical data registry is an interactive database that collects, organizes, and displays healthcare information. Clinical data registries are also sometimes. A medical device is any instrument, apparatus,. What Is A Medical Device Registry.
From www.interventionalconcepts.net
9 Steps for Medical Device Companies to Register and Sell their What Is A Medical Device Registry Clinical data registries are also sometimes. Medical devices need to be registered with mhra after they have been certified by an uk approved body, an eu notified body, or where they have. According to the medical devices regulations 2002 (si 2002 no 618, as amended) (uk mdr 2002), a medical device is described. Outcome registries are organised systems that use. What Is A Medical Device Registry.
From www.researchgate.net
(PDF) Design, implementation, and management of an international What Is A Medical Device Registry According to the medical devices regulations 2002 (si 2002 no 618, as amended) (uk mdr 2002), a medical device is described. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. This was an existing nhs england collection under the surgical devices. What is. What Is A Medical Device Registry.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU What Is A Medical Device Registry Outcome registries are organised systems that use observational methods to collect routine, uniform data on. This was an existing nhs england collection under the surgical devices. Medical devices need to be registered with mhra after they have been certified by an uk approved body, an eu notified body, or where they have. The medical devices and outcomes registry. A medical. What Is A Medical Device Registry.
From www.amazon.com
Medical Device Register 1996 (MEDICAL DEVICE REGISTER INTERNATIONAL What Is A Medical Device Registry According to the medical devices regulations 2002 (si 2002 no 618, as amended) (uk mdr 2002), a medical device is described. Medical devices need to be registered with mhra after they have been certified by an uk approved body, an eu notified body, or where they have. Outcome registries are organised systems that use observational methods to collect routine, uniform. What Is A Medical Device Registry.
From betebt.com
Medical Device Regulation Importance and Examples in APAC (2022) What Is A Medical Device Registry What is an outcome registry? This was an existing nhs england collection under the surgical devices. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. The medical devices and outcomes registry. Outcome registries are organised systems that use observational methods to collect routine,. What Is A Medical Device Registry.
From www.pacificbridgemedical.com
China Class 2 Device Registration Process & Timeline Chart What Is A Medical Device Registry Clinical data registries are also sometimes. What is an outcome registry? According to the medical devices regulations 2002 (si 2002 no 618, as amended) (uk mdr 2002), a medical device is described. A clinical data registry is an interactive database that collects, organizes, and displays healthcare information. This was an existing nhs england collection under the surgical devices. Outcome registries. What Is A Medical Device Registry.
From www.researchgate.net
(PDF) Developing a Policy and Procedure Framework and Manual for a What Is A Medical Device Registry According to the medical devices regulations 2002 (si 2002 no 618, as amended) (uk mdr 2002), a medical device is described. The medical devices and outcomes registry. What is an outcome registry? A clinical data registry is an interactive database that collects, organizes, and displays healthcare information. A medical device is any instrument, apparatus, appliance, software, material, or other article,. What Is A Medical Device Registry.
From www.regdesk.co
MHRA Guidance on Registration of Medical Devices RegDesk What Is A Medical Device Registry A clinical data registry is an interactive database that collects, organizes, and displays healthcare information. The medical devices and outcomes registry. What is an outcome registry? According to the medical devices regulations 2002 (si 2002 no 618, as amended) (uk mdr 2002), a medical device is described. Medical devices need to be registered with mhra after they have been certified. What Is A Medical Device Registry.
From corpbiz.io
Know Checklist for Documents Required For Medical Device Registration What Is A Medical Device Registry A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. The medical devices and outcomes registry. Clinical data registries are also sometimes. According to the medical devices regulations 2002 (si 2002 no 618, as amended) (uk mdr 2002), a medical device is described. What. What Is A Medical Device Registry.