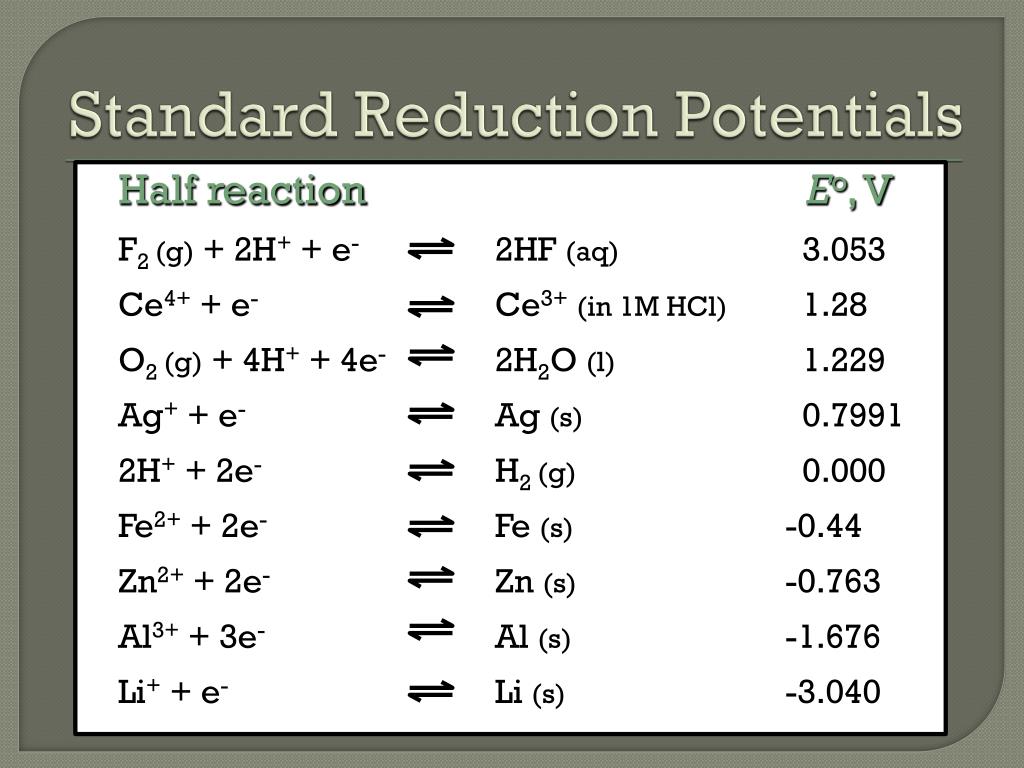
Standard Reduction Potential Hcl . 1 m for solutes) usually at 298.15 k; Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. The value of the reduction under standard conditions (1 bar or 1 atm for gases; Platinum, which is inert to the action of the 1 m hcl, is used as the electrode. Values are from the following sources: Use standard reduction potentials to determine the better oxidizing or. You may rewrite a reaction by replacing h + with h 3 o + and. Hydrogen gas at 1 atm is bubbled through 1 m hcl solution. The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. Reduction reactions in acidic solution are written using h + in place of h 3 o +. The following table provides e o for selected reduction reactions. Tabulated values used to calculate. Electrons on the surface of the electrode.
from www.slideserve.com
Use standard reduction potentials to determine the better oxidizing or. Hydrogen gas at 1 atm is bubbled through 1 m hcl solution. Electrons on the surface of the electrode. The following table provides e o for selected reduction reactions. The value of the reduction under standard conditions (1 bar or 1 atm for gases; The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. Reduction reactions in acidic solution are written using h + in place of h 3 o +. Values are from the following sources: You may rewrite a reaction by replacing h + with h 3 o + and. 1 m for solutes) usually at 298.15 k;
PPT Electrochemistry PowerPoint Presentation, free download ID6601383
Standard Reduction Potential Hcl Values are from the following sources: Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. You may rewrite a reaction by replacing h + with h 3 o + and. The value of the reduction under standard conditions (1 bar or 1 atm for gases; Platinum, which is inert to the action of the 1 m hcl, is used as the electrode. Reduction reactions in acidic solution are written using h + in place of h 3 o +. The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. Values are from the following sources: Use standard reduction potentials to determine the better oxidizing or. Hydrogen gas at 1 atm is bubbled through 1 m hcl solution. Electrons on the surface of the electrode. Tabulated values used to calculate. 1 m for solutes) usually at 298.15 k; The following table provides e o for selected reduction reactions.
From www.flinnsci.com
Standard Reduction Potential Charts for Chemistry Standard Reduction Potential Hcl Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. The following table provides e o for selected reduction reactions. Platinum, which is inert to the action of. Standard Reduction Potential Hcl.
From rayb78.github.io
Standard Reduction Potentials Chart Standard Reduction Potential Hcl You may rewrite a reaction by replacing h + with h 3 o + and. Values are from the following sources: Hydrogen gas at 1 atm is bubbled through 1 m hcl solution. Use standard reduction potentials to determine the better oxidizing or. Platinum, which is inert to the action of the 1 m hcl, is used as the electrode.. Standard Reduction Potential Hcl.
From www.pveducation.org
Standard Potential PVEducation Standard Reduction Potential Hcl Platinum, which is inert to the action of the 1 m hcl, is used as the electrode. Tabulated values used to calculate. 1 m for solutes) usually at 298.15 k; Hydrogen gas at 1 atm is bubbled through 1 m hcl solution. Reduction reactions in acidic solution are written using h + in place of h 3 o +. The. Standard Reduction Potential Hcl.
From www.slideserve.com
PPT CHEM1612 Pharmacy Week 9 Galvanic Cells PowerPoint Standard Reduction Potential Hcl You may rewrite a reaction by replacing h + with h 3 o + and. The following table provides e o for selected reduction reactions. Values are from the following sources: The value of the reduction under standard conditions (1 bar or 1 atm for gases; Reduction reactions in acidic solution are written using h + in place of h. Standard Reduction Potential Hcl.
From slideplayer.com
Electrochemistry Part III Reduction Potentials ppt download Standard Reduction Potential Hcl Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. Use standard reduction potentials to determine the better oxidizing or. Tabulated values used to calculate. Platinum, which is. Standard Reduction Potential Hcl.
From pressbooks.uiowa.edu
Topic 11 Appendix A Standard Reduction Potentials at 25ºC CHEM 1120 Standard Reduction Potential Hcl Tabulated values used to calculate. 1 m for solutes) usually at 298.15 k; Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. The following table provides e o for selected reduction reactions. The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode. Standard Reduction Potential Hcl.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Hcl Values are from the following sources: Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. The value of the reduction under standard conditions (1 bar or 1 atm for gases; Reduction reactions in acidic solution are written using h + in place of h 3 o +. Use standard reduction potentials. Standard Reduction Potential Hcl.
From saylordotorg.github.io
Standard Potentials Standard Reduction Potential Hcl 1 m for solutes) usually at 298.15 k; Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. The following table provides e o for selected reduction reactions. You may rewrite a reaction by replacing h + with h 3 o + and. Values are from the following sources: The value of. Standard Reduction Potential Hcl.
From saylordotorg.github.io
Standard Potentials Standard Reduction Potential Hcl Values are from the following sources: 1 m for solutes) usually at 298.15 k; Use standard reduction potentials to determine the better oxidizing or. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. Electrons on the surface of the electrode. Reduction reactions in acidic solution are written using h + in. Standard Reduction Potential Hcl.
From jonathancoles.z13.web.core.windows.net
Standard Reduction Potential Chart Standard Reduction Potential Hcl Hydrogen gas at 1 atm is bubbled through 1 m hcl solution. Reduction reactions in acidic solution are written using h + in place of h 3 o +. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. The value of the reduction under standard conditions (1 bar or 1 atm. Standard Reduction Potential Hcl.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Hcl 1 m for solutes) usually at 298.15 k; Reduction reactions in acidic solution are written using h + in place of h 3 o +. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. The values of standard electrode potentials are given in the table in volts relative to the standard. Standard Reduction Potential Hcl.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID6601383 Standard Reduction Potential Hcl Platinum, which is inert to the action of the 1 m hcl, is used as the electrode. You may rewrite a reaction by replacing h + with h 3 o + and. Electrons on the surface of the electrode. Tabulated values used to calculate. Reduction reactions in acidic solution are written using h + in place of h 3 o. Standard Reduction Potential Hcl.
From www.slideserve.com
PPT Product stabilizations in hydrolysis PowerPoint Presentation Standard Reduction Potential Hcl Platinum, which is inert to the action of the 1 m hcl, is used as the electrode. Tabulated values used to calculate. Reduction reactions in acidic solution are written using h + in place of h 3 o +. The following table provides e o for selected reduction reactions. Values are from the following sources: You may rewrite a reaction. Standard Reduction Potential Hcl.
From www.slideserve.com
PPT Chapter 18 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Hcl Platinum, which is inert to the action of the 1 m hcl, is used as the electrode. Values are from the following sources: The following table provides e o for selected reduction reactions. The value of the reduction under standard conditions (1 bar or 1 atm for gases; Reduction reactions in acidic solution are written using h + in place. Standard Reduction Potential Hcl.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Hcl Hydrogen gas at 1 atm is bubbled through 1 m hcl solution. Values are from the following sources: 1 m for solutes) usually at 298.15 k; Use standard reduction potentials to determine the better oxidizing or. You may rewrite a reaction by replacing h + with h 3 o + and. Tabulated values used to calculate. The value of the. Standard Reduction Potential Hcl.
From www.slideserve.com
PPT Redox reactions PowerPoint Presentation, free download ID3446507 Standard Reduction Potential Hcl Electrons on the surface of the electrode. Reduction reactions in acidic solution are written using h + in place of h 3 o +. You may rewrite a reaction by replacing h + with h 3 o + and. Platinum, which is inert to the action of the 1 m hcl, is used as the electrode. The values of standard. Standard Reduction Potential Hcl.
From chem.libretexts.org
6.5 Standard Reduction Potentials Chemistry LibreTexts Standard Reduction Potential Hcl You may rewrite a reaction by replacing h + with h 3 o + and. Hydrogen gas at 1 atm is bubbled through 1 m hcl solution. Electrons on the surface of the electrode. Platinum, which is inert to the action of the 1 m hcl, is used as the electrode. The values of standard electrode potentials are given in. Standard Reduction Potential Hcl.
From www.slideserve.com
PPT ELECTROCHEMISTRY Chapter 21 PowerPoint Presentation, free Standard Reduction Potential Hcl Reduction reactions in acidic solution are written using h + in place of h 3 o +. You may rewrite a reaction by replacing h + with h 3 o + and. Tabulated values used to calculate. Use standard reduction potentials to determine the better oxidizing or. Electrons on the surface of the electrode. The values of standard electrode potentials. Standard Reduction Potential Hcl.
From boisestate.pressbooks.pub
17.3 Standard Reduction Potentials General Chemistry 1 & 2 Standard Reduction Potential Hcl The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. Tabulated values used to calculate. Values are from the following sources: The following table provides e o for selected reduction reactions. The value of the reduction under standard conditions (1 bar or 1 atm for gases;. Standard Reduction Potential Hcl.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials Chemistry Standard Reduction Potential Hcl Tabulated values used to calculate. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. Reduction reactions in acidic solution are written using h + in place of h 3 o +. Values are from the following sources: Hydrogen gas at 1 atm is bubbled through 1 m hcl solution. 1 m. Standard Reduction Potential Hcl.
From www.docsity.com
Standard Reduction Potential Metabolic Biochemistry BIBC 102 Docsity Standard Reduction Potential Hcl Hydrogen gas at 1 atm is bubbled through 1 m hcl solution. Tabulated values used to calculate. The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. The following table provides e o for selected reduction reactions. Use standard reduction potentials to determine the better oxidizing. Standard Reduction Potential Hcl.
From www.youtube.com
Standard Reduction Potentials of Half Reactions Electrochemistry Standard Reduction Potential Hcl The following table provides e o for selected reduction reactions. Platinum, which is inert to the action of the 1 m hcl, is used as the electrode. Electrons on the surface of the electrode. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. Use standard reduction potentials to determine the better. Standard Reduction Potential Hcl.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Hcl The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. 1 m for solutes) usually at 298.15 k; Platinum, which is inert to the action of the 1. Standard Reduction Potential Hcl.
From hasyimmi.blogspot.com
Standard Reduction Potential Table Reduction Potentials in the Standard Reduction Potential Hcl You may rewrite a reaction by replacing h + with h 3 o + and. Platinum, which is inert to the action of the 1 m hcl, is used as the electrode. Values are from the following sources: Electrons on the surface of the electrode. Tabulated values used to calculate. Use standard reduction potentials to determine the better oxidizing or.. Standard Reduction Potential Hcl.
From www.numerade.com
SOLVED Given the following cell notation and list of standard Standard Reduction Potential Hcl Use standard reduction potentials to determine the better oxidizing or. The following table provides e o for selected reduction reactions. Hydrogen gas at 1 atm is bubbled through 1 m hcl solution. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. You may rewrite a reaction by replacing h + with. Standard Reduction Potential Hcl.
From www.slideserve.com
PPT The Oxidation States of Vanadium PowerPoint Presentation ID4176570 Standard Reduction Potential Hcl Tabulated values used to calculate. Use standard reduction potentials to determine the better oxidizing or. The following table provides e o for selected reduction reactions. You may rewrite a reaction by replacing h + with h 3 o + and. The value of the reduction under standard conditions (1 bar or 1 atm for gases; Reduction reactions in acidic solution. Standard Reduction Potential Hcl.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Hcl Reduction reactions in acidic solution are written using h + in place of h 3 o +. The following table provides e o for selected reduction reactions. Use standard reduction potentials to determine the better oxidizing or. 1 m for solutes) usually at 298.15 k; You may rewrite a reaction by replacing h + with h 3 o + and.. Standard Reduction Potential Hcl.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Hcl Platinum, which is inert to the action of the 1 m hcl, is used as the electrode. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. Hydrogen gas at 1 atm is bubbled through 1 m hcl solution. 1 m for solutes) usually at 298.15 k; The value of the reduction. Standard Reduction Potential Hcl.
From www.slideserve.com
PPT Chemistry 1011 PowerPoint Presentation, free download ID5404690 Standard Reduction Potential Hcl The following table provides e o for selected reduction reactions. Values are from the following sources: Tabulated values used to calculate. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. Platinum, which is inert to the action of the 1 m hcl, is used as the electrode. Hydrogen gas at 1. Standard Reduction Potential Hcl.
From www.slideserve.com
PPT Cell Potentials at Standard Conditions PowerPoint Presentation Standard Reduction Potential Hcl Values are from the following sources: Electrons on the surface of the electrode. Hydrogen gas at 1 atm is bubbled through 1 m hcl solution. Use standard reduction potentials to determine the better oxidizing or. The following table provides e o for selected reduction reactions. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination. Standard Reduction Potential Hcl.
From chem.libretexts.org
17.3 Standard Reduction Potentials Chemistry LibreTexts Standard Reduction Potential Hcl Electrons on the surface of the electrode. The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. Hydrogen gas at 1 atm is bubbled through 1 m hcl solution. Values are from the following sources: Reduction reactions in acidic solution are written using h + in. Standard Reduction Potential Hcl.
From www.slideserve.com
PPT Electrochemistry & Virus Templated Electrodes PowerPoint Standard Reduction Potential Hcl Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. The following table provides e o for selected reduction reactions. Tabulated values used to calculate. Hydrogen gas at 1 atm is bubbled through 1 m hcl solution. Electrons on the surface of the electrode. The values of standard electrode potentials are given. Standard Reduction Potential Hcl.
From www.slideserve.com
PPT The oxidation states of vanadium PowerPoint Presentation ID467926 Standard Reduction Potential Hcl Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. 1 m for solutes) usually at 298.15 k; Values are from the following sources: You may rewrite a reaction by replacing h + with h 3 o + and. Hydrogen gas at 1 atm is bubbled through 1 m hcl solution. The. Standard Reduction Potential Hcl.
From testbook.com
Reduction Potential Measurement, Half Cells, Differences & More Standard Reduction Potential Hcl Values are from the following sources: Reduction reactions in acidic solution are written using h + in place of h 3 o +. You may rewrite a reaction by replacing h + with h 3 o + and. Electrons on the surface of the electrode. The value of the reduction under standard conditions (1 bar or 1 atm for gases;. Standard Reduction Potential Hcl.
From ar.inspiredpencil.com
Standard Reduction Potential Table Standard Reduction Potential Hcl The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. Use standard reduction potentials to determine the better oxidizing or. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. Platinum, which is inert to the action of. Standard Reduction Potential Hcl.