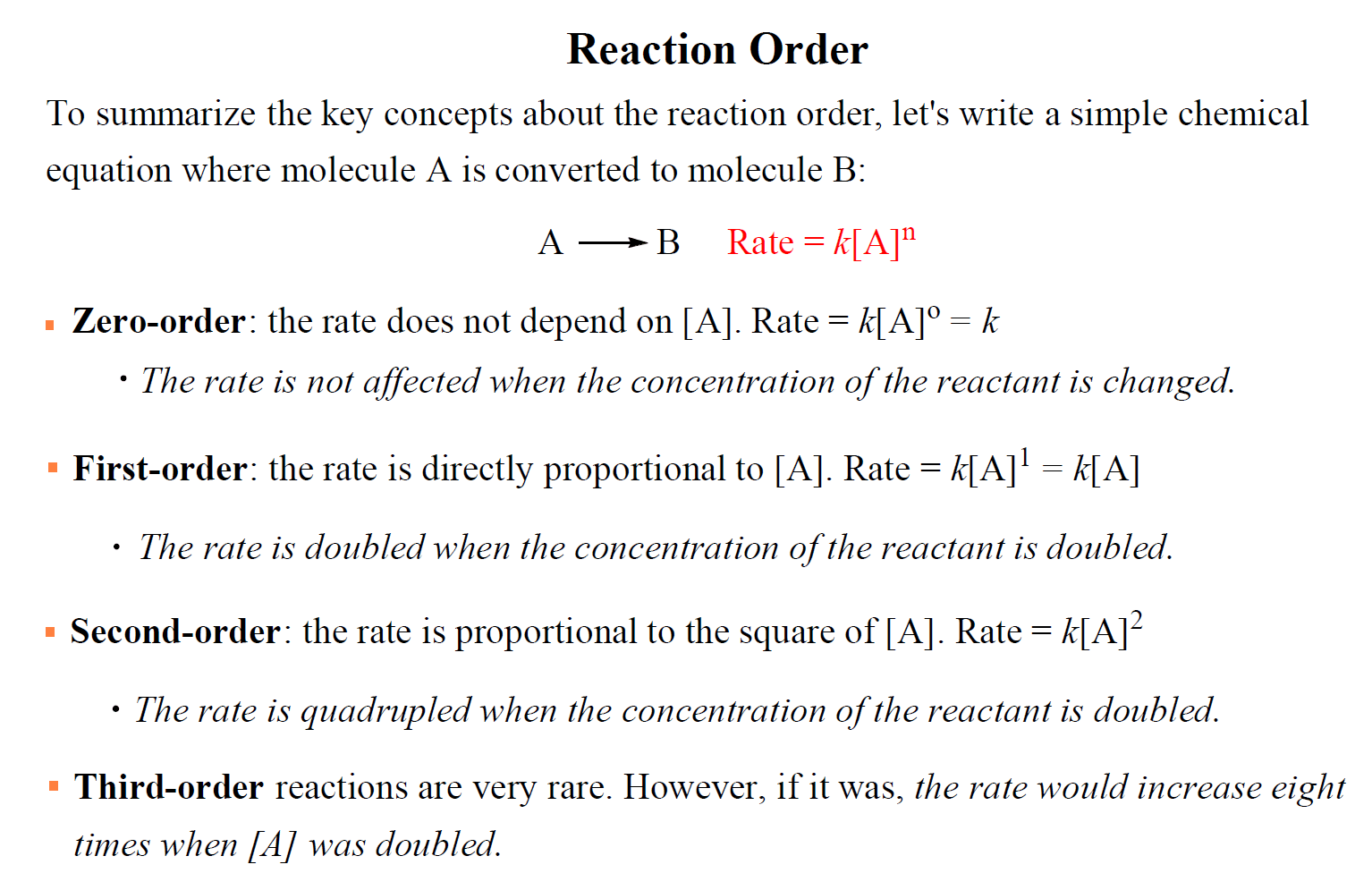
The Rate Constant Of First Order Reaction Becomes 5 Times . Where k is the rate constant and x is the order of reaction with respect to reactants a and b. What is its rate constant? The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. The rate constant may be found experimentally, using the molar concentrations of the reactants and the order of reaction.
from general.chemistrysteps.com
The rate constant may be found experimentally, using the molar concentrations of the reactants and the order of reaction. The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. What is its rate constant? The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. Where k is the rate constant and x is the order of reaction with respect to reactants a and b.
Rate Law and Reaction Order Chemistry Steps
The Rate Constant Of First Order Reaction Becomes 5 Times The rate constant may be found experimentally, using the molar concentrations of the reactants and the order of reaction. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Where k is the rate constant and x is the order of reaction with respect to reactants a and b. The rate constant may be found experimentally, using the molar concentrations of the reactants and the order of reaction. What is its rate constant? The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k.
From askfilo.com
57. The rate constant of a first order reaction is 3×10−3 s−1. Calculate The Rate Constant Of First Order Reaction Becomes 5 Times The rate constant may be found experimentally, using the molar concentrations of the reactants and the order of reaction. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Where k is the rate constant and x is the order of reaction with respect to. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.brainkart.com
Rate equation for first order reactions The Rate Constant Of First Order Reaction Becomes 5 Times What is its rate constant? The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate constant may be found experimentally, using the molar concentrations of the reactants and the order of reaction. The rate constant of a first order reaction becomes 5 times. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.youtube.com
first order reaction rate calculation example YouTube The Rate Constant Of First Order Reaction Becomes 5 Times The rate constant may be found experimentally, using the molar concentrations of the reactants and the order of reaction. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate constant of a first order reaction becomes 5 times when the temperature is raised. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.slideserve.com
PPT Rate laws (2) PowerPoint Presentation, free download ID2351800 The Rate Constant Of First Order Reaction Becomes 5 Times The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. What is its rate constant? The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Where k is the rate constant and x. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.brainkart.com
Rate equation for first order reactions The Rate Constant Of First Order Reaction Becomes 5 Times What is its rate constant? The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. The rate constant may be found experimentally, using the molar concentrations of the reactants and the order of reaction. The rate constant, k, is a proportionality constant that indicates the relationship between the. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.chegg.com
Solved Extracting rate constants of firstorder reactions The Rate Constant Of First Order Reaction Becomes 5 Times Where k is the rate constant and x is the order of reaction with respect to reactants a and b. The rate constant may be found experimentally, using the molar concentrations of the reactants and the order of reaction. The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.meritnation.com
The rate constant of a first order reaction increases from 2 X 102 to The Rate Constant Of First Order Reaction Becomes 5 Times The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. The rate constant may be found experimentally, using the molar concentrations of the. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.slideserve.com
PPT Chapter 15 Chemical The Rates of Chemical Reactions The Rate Constant Of First Order Reaction Becomes 5 Times The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. What is its rate constant? The rate constant of a first order reaction. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.youtube.com
Integrated Rate Equation for first Order Reaction First Order The Rate Constant Of First Order Reaction Becomes 5 Times The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Where k is the rate constant and x is the order of reaction. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.youtube.com
Intro to Rate Laws, Rate Constants, Reaction Order Chemistry Tutorial The Rate Constant Of First Order Reaction Becomes 5 Times The rate constant may be found experimentally, using the molar concentrations of the reactants and the order of reaction. What is its rate constant? The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Where k is the rate constant and x is the order. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.youtube.com
Quickvideo Calculating rate constant from a firstorder reaction The Rate Constant Of First Order Reaction Becomes 5 Times The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. What is its rate constant? The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate constant of a first order reaction. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.researchgate.net
Firstorder reaction rate constants (k obs ) and initial reaction rates The Rate Constant Of First Order Reaction Becomes 5 Times The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. Where k is the rate constant and x is the order of reaction. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.biologyonline.com
Firstorder Definition and Examples Biology Online Dictionary The Rate Constant Of First Order Reaction Becomes 5 Times The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Where k is the rate constant and x is the order of reaction with respect to reactants a and b. The rate constant of a first order reaction becomes 5 times when the temperature is. The Rate Constant Of First Order Reaction Becomes 5 Times.
From study.com
First Order Reaction & Rate Law Definition, Equation & Examples The Rate Constant Of First Order Reaction Becomes 5 Times The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.chegg.com
Solved The rate constant for this firstorder reaction is The Rate Constant Of First Order Reaction Becomes 5 Times What is its rate constant? The rate constant may be found experimentally, using the molar concentrations of the reactants and the order of reaction. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Where k is the rate constant and x is the order. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.youtube.com
Integrated rate equation for first order reaction YouTube The Rate Constant Of First Order Reaction Becomes 5 Times The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Where k is the rate constant and x is the order of reaction with respect to reactants a and b. The rate constant of a first order reaction becomes 5 times when the temperature is. The Rate Constant Of First Order Reaction Becomes 5 Times.
From newbedev.com
Chemistry Formula for rate constant for the first order reaction The Rate Constant Of First Order Reaction Becomes 5 Times The rate constant may be found experimentally, using the molar concentrations of the reactants and the order of reaction. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate constant of a first order reaction becomes 5 times when the temperature is raised. The Rate Constant Of First Order Reaction Becomes 5 Times.
From general.chemistrysteps.com
Rate Law and Reaction Order Chemistry Steps The Rate Constant Of First Order Reaction Becomes 5 Times The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. Where k is the rate constant and x is the order of reaction with respect to reactants a and b. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.slideserve.com
PPT Reaction Rates PowerPoint Presentation, free download ID6090305 The Rate Constant Of First Order Reaction Becomes 5 Times What is its rate constant? The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. The rate constant may be found experimentally, using the molar concentrations of the reactants and the order of reaction. The rate constant, k, is a proportionality constant that indicates the relationship between the. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.toppr.com
The rate constant of a first order reaction 5 times when the The Rate Constant Of First Order Reaction Becomes 5 Times The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. Where k is the rate constant and x is the order of reaction with respect to reactants a and b. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants. The Rate Constant Of First Order Reaction Becomes 5 Times.
From mavink.com
First Order Reaction Plot The Rate Constant Of First Order Reaction Becomes 5 Times What is its rate constant? The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. The rate constant may be found experimentally, using the molar concentrations. The Rate Constant Of First Order Reaction Becomes 5 Times.
From byjus.com
What is the unit of rate constant for first order reaction The Rate Constant Of First Order Reaction Becomes 5 Times Where k is the rate constant and x is the order of reaction with respect to reactants a and b. The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. The rate constant may be found experimentally, using the molar concentrations of the reactants and the order of. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.youtube.com
Rate constant for first order reaction of gas phase reaction YouTube The Rate Constant Of First Order Reaction Becomes 5 Times What is its rate constant? The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. The rate constant of a first order reaction. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.researchgate.net
How to calculate rate constant for first order reaction? ResearchGate The Rate Constant Of First Order Reaction Becomes 5 Times The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.chegg.com
Solved The rate constant of a firstorder reaction is The Rate Constant Of First Order Reaction Becomes 5 Times The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. The rate constant may be found experimentally, using the molar concentrations of the reactants and the order of reaction. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID6630235 The Rate Constant Of First Order Reaction Becomes 5 Times The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. Where k is the rate constant and x is the order of reaction with respect to reactants a and b. The rate constant may be found experimentally, using the molar concentrations of the reactants and the order of. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.youtube.com
The First Order Integrated Rate Law and Half Life (Part 4) YouTube The Rate Constant Of First Order Reaction Becomes 5 Times What is its rate constant? Where k is the rate constant and x is the order of reaction with respect to reactants a and b. The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. The rate constant, k, is a proportionality constant that indicates the relationship between. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.slideserve.com
PPT Reaction Rates PowerPoint Presentation, free download ID6090305 The Rate Constant Of First Order Reaction Becomes 5 Times What is its rate constant? The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. Where k is the rate constant and x is the order of reaction with respect to reactants a and b. The rate constant, k, is a proportionality constant that indicates the relationship between. The Rate Constant Of First Order Reaction Becomes 5 Times.
From askfilo.com
The rate constant of a first order reaction depends on the Filo The Rate Constant Of First Order Reaction Becomes 5 Times The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. The rate constant may be found experimentally, using the molar concentrations of the. The Rate Constant Of First Order Reaction Becomes 5 Times.
From 9to5science.com
[Solved] Formula for rate constant for the first order 9to5Science The Rate Constant Of First Order Reaction Becomes 5 Times Where k is the rate constant and x is the order of reaction with respect to reactants a and b. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate constant of a first order reaction becomes 5 times when the temperature is. The Rate Constant Of First Order Reaction Becomes 5 Times.
From chemistryguru.com.sg
Rate Equation and Order of Reaction The Rate Constant Of First Order Reaction Becomes 5 Times The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. What is its rate constant? The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. The rate constant, k, is a proportionality constant that indicates the. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.toppr.com
For a first order reaction, the plot of t against log[a x] gives a The Rate Constant Of First Order Reaction Becomes 5 Times What is its rate constant? The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Where k is the rate constant and x. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.coursehero.com
[Solved] A certain firstorder reaction has a rate constant of The Rate Constant Of First Order Reaction Becomes 5 Times The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. The rate constant may be found experimentally, using the molar concentrations of the reactants and the order of reaction. The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k. The Rate Constant Of First Order Reaction Becomes 5 Times.
From kunduz.com
[ANSWERED] If the rate constant for a first order re... Physical The Rate Constant Of First Order Reaction Becomes 5 Times Where k is the rate constant and x is the order of reaction with respect to reactants a and b. The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350. The Rate Constant Of First Order Reaction Becomes 5 Times.
From www.youtube.com
The rate constant for a first order reaction six times when the The Rate Constant Of First Order Reaction Becomes 5 Times The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. What is its rate constant? The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. The rate constant of a first order reaction. The Rate Constant Of First Order Reaction Becomes 5 Times.