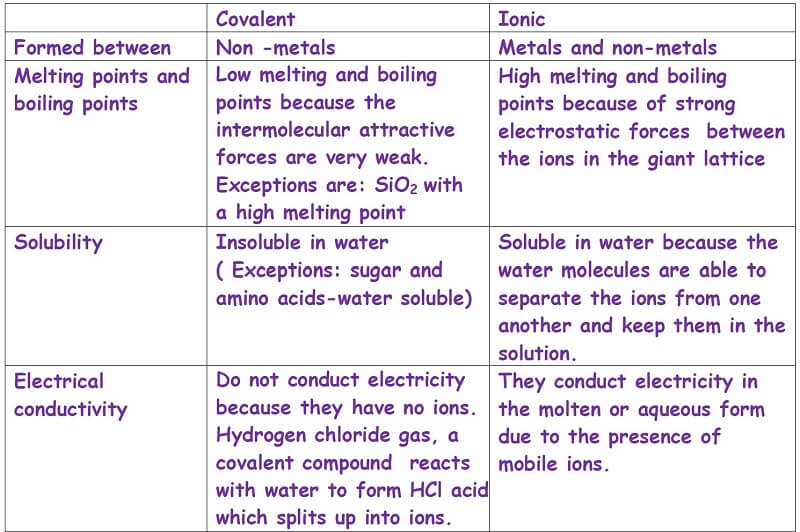
Do Ionic Or Covalent Bonds Have Higher Melting Points . Ionic compounds have high melting points. Ionic compounds are hard and brittle. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. The melting point is a macroscopic property of a compund or element,. For example, the molecule carbon. Neither ionic nor covalent bonds have a melting point. Ionic compounds have high melting points. Ionic compounds are hard and brittle. This generally leads to low melting points for covalent solids, and high melting points for ionic solids. Covalent bonds are highly stable bonds with. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. All ionic compounds have a high melting point and boiling point because many strong ionic bonds need to be broken.
from www.smartexamresources.com
All ionic compounds have a high melting point and boiling point because many strong ionic bonds need to be broken. This generally leads to low melting points for covalent solids, and high melting points for ionic solids. Ionic compounds have high melting points. Covalent bonds are highly stable bonds with. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. Ionic compounds dissociate into ions when dissolved in water. The melting point is a macroscopic property of a compund or element,. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Neither ionic nor covalent bonds have a melting point. Ionic compounds are hard and brittle.
IGCSE Notes Ions And Ionic Bonds Smart Exam Resources
Do Ionic Or Covalent Bonds Have Higher Melting Points The melting point is a macroscopic property of a compund or element,. This generally leads to low melting points for covalent solids, and high melting points for ionic solids. Ionic compounds are hard and brittle. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Ionic compounds have high melting points. Ionic compounds have high melting points. All ionic compounds have a high melting point and boiling point because many strong ionic bonds need to be broken. Covalent bonds are highly stable bonds with. For example, the molecule carbon. The melting point is a macroscopic property of a compund or element,. Ionic compounds are hard and brittle. Ionic compounds dissociate into ions when dissolved in water. Neither ionic nor covalent bonds have a melting point. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials.
From quizlet.com
Ionic and Covalent Bonds Diagram Quizlet Do Ionic Or Covalent Bonds Have Higher Melting Points Neither ionic nor covalent bonds have a melting point. This generally leads to low melting points for covalent solids, and high melting points for ionic solids. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds have high melting points. The melting point is a macroscopic property of a compund or element,. Solutions of ionic compounds and melted ionic. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From mavink.com
Melting Point Chart Of Compounds Do Ionic Or Covalent Bonds Have Higher Melting Points Ionic compounds dissociate into ions when dissolved in water. All ionic compounds have a high melting point and boiling point because many strong ionic bonds need to be broken. For example, the molecule carbon. The melting point is a macroscopic property of a compund or element,. Ionic compounds have high melting points. Neither ionic nor covalent bonds have a melting. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From www.masterorganicchemistry.com
Ionic and Covalent Bonding Master Organic Chemistry Do Ionic Or Covalent Bonds Have Higher Melting Points Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. All ionic compounds have a high melting point and boiling point because many strong ionic bonds need to be broken. Ionic compounds are hard and brittle. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Ionic compounds have high melting points.. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From www.nagwa.com
Question Video Determining Why Covalent Compounds Have Low Melting and Do Ionic Or Covalent Bonds Have Higher Melting Points Neither ionic nor covalent bonds have a melting point. For example, the molecule carbon. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds have high melting points. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. This generally leads to low melting points for covalent solids, and high melting points for ionic. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From www.youtube.com
Melting Points of Ionic Compounds YouTube Do Ionic Or Covalent Bonds Have Higher Melting Points Ionic compounds are hard and brittle. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. Ionic compounds are hard and brittle. All ionic compounds have a high melting point and boiling point because many strong ionic bonds need to be broken. Ionic compounds tend to be crystalline structures with high melting points that are water soluble.. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From www.slideserve.com
PPT The Structure of Matter PowerPoint Presentation, free download Do Ionic Or Covalent Bonds Have Higher Melting Points Ionic compounds dissociate into ions when dissolved in water. For example, the molecule carbon. This generally leads to low melting points for covalent solids, and high melting points for ionic solids. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. The. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica Do Ionic Or Covalent Bonds Have Higher Melting Points Neither ionic nor covalent bonds have a melting point. Ionic compounds are hard and brittle. Ionic compounds have high melting points. Covalent bonds are highly stable bonds with. This generally leads to low melting points for covalent solids, and high melting points for ionic solids. For example, the molecule carbon. Ionic compounds have high melting points. Ionic compounds are hard. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From www.slideserve.com
PPT Properties of Ionic Bonds PowerPoint Presentation, free download Do Ionic Or Covalent Bonds Have Higher Melting Points This generally leads to low melting points for covalent solids, and high melting points for ionic solids. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. Neither ionic nor covalent bonds have a melting point. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Ionic compounds are hard and brittle.. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From chem.libretexts.org
6.2 Comparing Ionic and Molecular Substances Chemistry LibreTexts Do Ionic Or Covalent Bonds Have Higher Melting Points Ionic compounds dissociate into ions when dissolved in water. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Covalent bonds are highly stable bonds with. Ionic compounds are hard and brittle. Ionic compounds have high melting points. The melting point is a macroscopic property of a compund or element,. Ionic compounds are hard and. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From www.youtube.com
Properties of Ionic and Covalent Compounds YouTube Do Ionic Or Covalent Bonds Have Higher Melting Points All ionic compounds have a high melting point and boiling point because many strong ionic bonds need to be broken. The melting point is a macroscopic property of a compund or element,. Covalent bonds are highly stable bonds with. For example, the molecule carbon. This generally leads to low melting points for covalent solids, and high melting points for ionic. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From msestudent.com
17 Metals With the Highest Melting Points (and Why) Materials Science Do Ionic Or Covalent Bonds Have Higher Melting Points Ionic compounds are hard and brittle. Ionic compounds are hard and brittle. For example, the molecule carbon. This generally leads to low melting points for covalent solids, and high melting points for ionic solids. Ionic compounds have high melting points. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Ionic compounds dissociate into ions. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From www.breakingatom.com
Ionic Properties Do Ionic Or Covalent Bonds Have Higher Melting Points Ionic compounds have high melting points. Ionic compounds have high melting points. The melting point is a macroscopic property of a compund or element,. Covalent bonds are highly stable bonds with. Ionic compounds are hard and brittle. For example, the molecule carbon. Neither ionic nor covalent bonds have a melting point. Solutions of ionic compounds and melted ionic compounds conduct. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From telgurus.co.uk
Why Do Ionic Compounds Have High Melting Points And Boiling Points? Do Ionic Or Covalent Bonds Have Higher Melting Points Neither ionic nor covalent bonds have a melting point. For example, the molecule carbon. All ionic compounds have a high melting point and boiling point because many strong ionic bonds need to be broken. Ionic compounds have high melting points. This generally leads to low melting points for covalent solids, and high melting points for ionic solids. Ionic compounds dissociate. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From studylib.net
Ionic, Covalent, and Metallic Bonding Summary Do Ionic Or Covalent Bonds Have Higher Melting Points Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Neither ionic nor covalent bonds have a melting point. For example, the molecule carbon. Ionic compounds are hard and brittle. Ionic compounds dissociate into ions when dissolved in water. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. Ionic compounds have. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From www.chemistrylearner.com
Ionic, Covalent, and Metallic Bonds Differences and Similarities Do Ionic Or Covalent Bonds Have Higher Melting Points For example, the molecule carbon. All ionic compounds have a high melting point and boiling point because many strong ionic bonds need to be broken. Neither ionic nor covalent bonds have a melting point. Ionic compounds are hard and brittle. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Ionic compounds have high melting. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From www.slideserve.com
PPT Chapter 9 Chemical Bonding I Lewis Theory PowerPoint Do Ionic Or Covalent Bonds Have Higher Melting Points Ionic compounds tend to be crystalline structures with high melting points that are water soluble. All ionic compounds have a high melting point and boiling point because many strong ionic bonds need to be broken. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. The melting point is a macroscopic property of a compund or element,.. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From pediaa.com
Difference Between Covalent and Ionic Bonds Do Ionic Or Covalent Bonds Have Higher Melting Points This generally leads to low melting points for covalent solids, and high melting points for ionic solids. Ionic compounds are hard and brittle. Ionic compounds have high melting points. The melting point is a macroscopic property of a compund or element,. All ionic compounds have a high melting point and boiling point because many strong ionic bonds need to be. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From www.expii.com
Ionic Bond — Formation & Compounds Expii Do Ionic Or Covalent Bonds Have Higher Melting Points Ionic compounds are hard and brittle. This generally leads to low melting points for covalent solids, and high melting points for ionic solids. Ionic compounds are hard and brittle. The melting point is a macroscopic property of a compund or element,. For example, the molecule carbon. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. Neither. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From www.expii.com
Ionic Bond — Formation & Compounds Expii Do Ionic Or Covalent Bonds Have Higher Melting Points Ionic compounds are hard and brittle. For example, the molecule carbon. Covalent bonds are highly stable bonds with. Ionic compounds have high melting points. All ionic compounds have a high melting point and boiling point because many strong ionic bonds need to be broken. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. This generally leads. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From www.youtube.com
Melting Point Trends of Ionic Compounds YouTube Do Ionic Or Covalent Bonds Have Higher Melting Points Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Neither ionic nor covalent bonds have a melting point. Ionic compounds are hard and brittle. Ionic compounds dissociate into ions when dissolved in water. The melting point is a macroscopic property of a compund or element,. This generally leads to low melting points for covalent. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica Do Ionic Or Covalent Bonds Have Higher Melting Points Neither ionic nor covalent bonds have a melting point. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. Covalent bonds are highly stable bonds with. Ionic compounds are hard and brittle. All ionic compounds have a high melting point and boiling point because many strong ionic bonds need to be broken. Ionic compounds have high melting. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From socratic.org
Is an ionic bond stronger than a comparable covalent bond? If not, then Do Ionic Or Covalent Bonds Have Higher Melting Points Neither ionic nor covalent bonds have a melting point. Ionic compounds are hard and brittle. For example, the molecule carbon. Ionic compounds are hard and brittle. All ionic compounds have a high melting point and boiling point because many strong ionic bonds need to be broken. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds tend to be. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Do Ionic Or Covalent Bonds Have Higher Melting Points For example, the molecule carbon. The melting point is a macroscopic property of a compund or element,. All ionic compounds have a high melting point and boiling point because many strong ionic bonds need to be broken. This generally leads to low melting points for covalent solids, and high melting points for ionic solids. Neither ionic nor covalent bonds have. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From www.shalom-education.com
Properties of Ionic Compounds GCSE Chemistry Revision Do Ionic Or Covalent Bonds Have Higher Melting Points The melting point is a macroscopic property of a compund or element,. Neither ionic nor covalent bonds have a melting point. Covalent bonds are highly stable bonds with. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. Ionic compounds have high melting points. All ionic compounds have a high melting point and boiling point because many. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From www.myxxgirl.com
Ionic Bonding Melting Point My XXX Hot Girl Do Ionic Or Covalent Bonds Have Higher Melting Points Covalent bonds are highly stable bonds with. This generally leads to low melting points for covalent solids, and high melting points for ionic solids. Ionic compounds dissociate into ions when dissolved in water. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. Ionic compounds are hard and brittle. The melting point is a macroscopic property of. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From wagine.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Do Ionic Or Covalent Bonds Have Higher Melting Points Ionic compounds dissociate into ions when dissolved in water. All ionic compounds have a high melting point and boiling point because many strong ionic bonds need to be broken. Ionic compounds are hard and brittle. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. Ionic compounds have high melting points. Covalent bonds are highly stable bonds. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From www.smartexamresources.com
IGCSE Notes Ions And Ionic Bonds Smart Exam Resources Do Ionic Or Covalent Bonds Have Higher Melting Points Ionic compounds tend to be crystalline structures with high melting points that are water soluble. The melting point is a macroscopic property of a compund or element,. Neither ionic nor covalent bonds have a melting point. Ionic compounds are hard and brittle. Covalent bonds are highly stable bonds with. Ionic compounds have high melting points. All ionic compounds have a. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From www.youtube.com
GCSE Chemistry 19 Why do Ionic Compounds have High Melting Points Do Ionic Or Covalent Bonds Have Higher Melting Points Ionic compounds have high melting points. All ionic compounds have a high melting point and boiling point because many strong ionic bonds need to be broken. Neither ionic nor covalent bonds have a melting point. Ionic compounds have high melting points. Ionic compounds are hard and brittle. Ionic compounds are hard and brittle. Covalent bonds are highly stable bonds with.. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From www.sliderbase.com
Molecules with Expanded Valence Shells Do Ionic Or Covalent Bonds Have Higher Melting Points Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Ionic compounds have high melting points. The melting point is a macroscopic property of a compund or element,. For example, the molecule carbon. Covalent bonds are highly stable bonds with. All ionic. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts Do Ionic Or Covalent Bonds Have Higher Melting Points This generally leads to low melting points for covalent solids, and high melting points for ionic solids. Ionic compounds are hard and brittle. The melting point is a macroscopic property of a compund or element,. For example, the molecule carbon. Neither ionic nor covalent bonds have a melting point. Ionic compounds have high melting points. Ionic compounds tend to be. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From byjus.com
Why are ionic solids have lesser melting point than covalent solids Do Ionic Or Covalent Bonds Have Higher Melting Points Ionic compounds are hard and brittle. Neither ionic nor covalent bonds have a melting point. All ionic compounds have a high melting point and boiling point because many strong ionic bonds need to be broken. Covalent bonds are highly stable bonds with. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. Ionic compounds have high melting. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From www.slideshare.net
Chem matters ch7_covalent_bonding Do Ionic Or Covalent Bonds Have Higher Melting Points This generally leads to low melting points for covalent solids, and high melting points for ionic solids. Ionic compounds dissociate into ions when dissolved in water. For example, the molecule carbon. Ionic compounds are hard and brittle. Ionic compounds have high melting points. Ionic compounds are hard and brittle. Ionic compounds tend to be crystalline structures with high melting points. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From www.pinterest.com
Properties of Ionic Compounds Ionic compound, Ionic, Compounds Do Ionic Or Covalent Bonds Have Higher Melting Points Ionic compounds tend to be crystalline structures with high melting points that are water soluble. This generally leads to low melting points for covalent solids, and high melting points for ionic solids. Ionic compounds have high melting points. Neither ionic nor covalent bonds have a melting point. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials.. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From www.youtube.com
Metallic, Ionic, and Molecular Solids Explained with Do Ionic Or Covalent Bonds Have Higher Melting Points Ionic compounds are hard and brittle. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds have high melting points. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. All ionic compounds have a high melting point and boiling point because many strong ionic bonds need to be broken. Ionic compounds tend to be crystalline. Do Ionic Or Covalent Bonds Have Higher Melting Points.
From chemistryguru.com.sg
Melting Point of Period 3 Oxides Do Ionic Or Covalent Bonds Have Higher Melting Points Ionic compounds are hard and brittle. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Covalent bonds are highly stable bonds with. Ionic compounds have high melting points. For example, the molecule carbon. All ionic compounds have a high melting point and boiling point because many strong ionic bonds need to be broken. Ionic. Do Ionic Or Covalent Bonds Have Higher Melting Points.