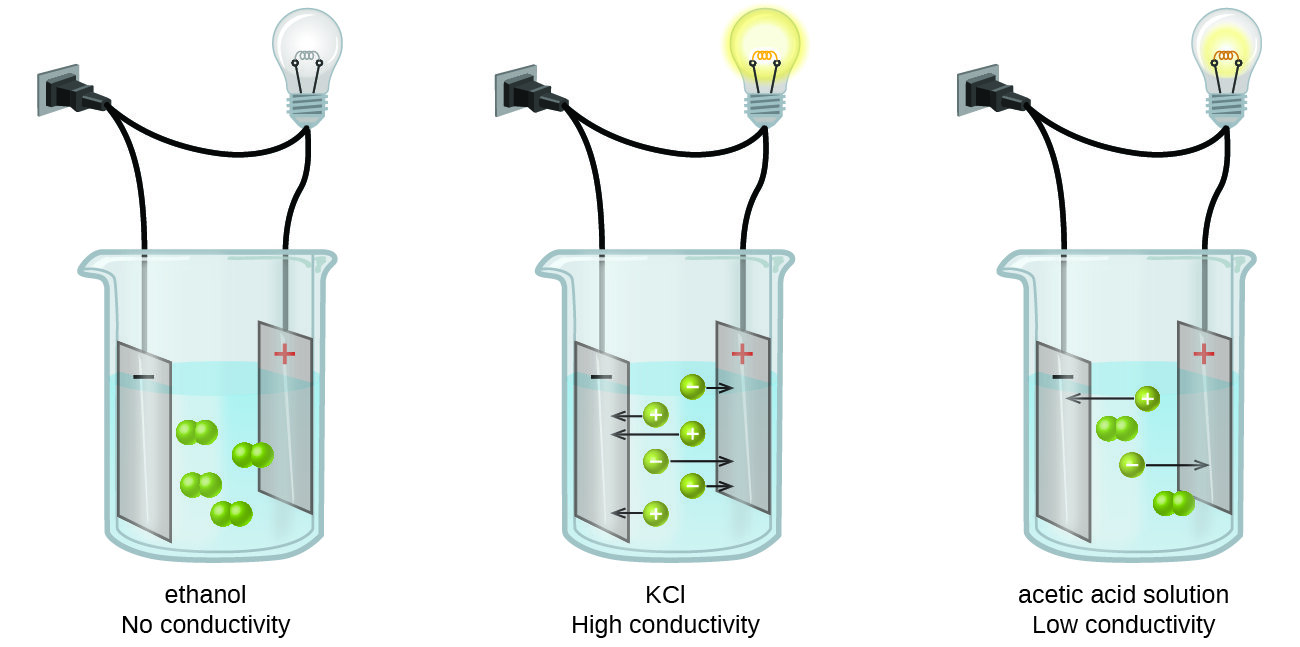
How To Measure Ion Conductivity . To interpret a chemical reaction by observing aqueous solution conductivity. the determination of ph value, conductivity and related parameters such as ion concentration, resistivity, and salinity, are. to determine of the solution is a strong or weak electrolyte. As the ranges in aqueous solutions are usually small, the. conductivity is measured using a conductivity meter and probe. the equivalent conductance increases with the charge number and decreases with the size. The expected values can differ from. conductivity meters measure the ion capacity in aqueous solution to carry electrical current. A voltage is applied between two electrodes inside the conductivity. conductivity is a measure of how well a solution conducts electricity. To carry a current a solution must contain charged.
from group.chem.iastate.edu
the determination of ph value, conductivity and related parameters such as ion concentration, resistivity, and salinity, are. A voltage is applied between two electrodes inside the conductivity. conductivity meters measure the ion capacity in aqueous solution to carry electrical current. To carry a current a solution must contain charged. the equivalent conductance increases with the charge number and decreases with the size. to determine of the solution is a strong or weak electrolyte. To interpret a chemical reaction by observing aqueous solution conductivity. As the ranges in aqueous solutions are usually small, the. conductivity is measured using a conductivity meter and probe. conductivity is a measure of how well a solution conducts electricity.
Conductivity Meter Holme Research Group Iowa State University
How To Measure Ion Conductivity to determine of the solution is a strong or weak electrolyte. the equivalent conductance increases with the charge number and decreases with the size. conductivity is a measure of how well a solution conducts electricity. A voltage is applied between two electrodes inside the conductivity. The expected values can differ from. To carry a current a solution must contain charged. conductivity is measured using a conductivity meter and probe. To interpret a chemical reaction by observing aqueous solution conductivity. to determine of the solution is a strong or weak electrolyte. the determination of ph value, conductivity and related parameters such as ion concentration, resistivity, and salinity, are. As the ranges in aqueous solutions are usually small, the. conductivity meters measure the ion capacity in aqueous solution to carry electrical current.
From blog.hannainst.com
8 Common Mistakes When Taking Conductivity Measurements How To Measure Ion Conductivity conductivity is a measure of how well a solution conducts electricity. the determination of ph value, conductivity and related parameters such as ion concentration, resistivity, and salinity, are. the equivalent conductance increases with the charge number and decreases with the size. A voltage is applied between two electrodes inside the conductivity. to determine of the solution. How To Measure Ion Conductivity.
From www.researchgate.net
Ionic conductivity measurement with chemical modifications and physical How To Measure Ion Conductivity the determination of ph value, conductivity and related parameters such as ion concentration, resistivity, and salinity, are. To interpret a chemical reaction by observing aqueous solution conductivity. As the ranges in aqueous solutions are usually small, the. To carry a current a solution must contain charged. conductivity is a measure of how well a solution conducts electricity. A. How To Measure Ion Conductivity.
From byjus.com
Conduction of Electricity in Liquids Electrolysis, Reduction at How To Measure Ion Conductivity As the ranges in aqueous solutions are usually small, the. The expected values can differ from. conductivity is measured using a conductivity meter and probe. To carry a current a solution must contain charged. conductivity meters measure the ion capacity in aqueous solution to carry electrical current. the determination of ph value, conductivity and related parameters such. How To Measure Ion Conductivity.
From www.science.org
Enhancing ionic conductivity in solid electrolyte by relocating How To Measure Ion Conductivity conductivity is measured using a conductivity meter and probe. A voltage is applied between two electrodes inside the conductivity. To carry a current a solution must contain charged. The expected values can differ from. conductivity meters measure the ion capacity in aqueous solution to carry electrical current. the determination of ph value, conductivity and related parameters such. How To Measure Ion Conductivity.
From www.researchgate.net
Characterization method for separating the ionic conductance from the How To Measure Ion Conductivity The expected values can differ from. To interpret a chemical reaction by observing aqueous solution conductivity. the determination of ph value, conductivity and related parameters such as ion concentration, resistivity, and salinity, are. to determine of the solution is a strong or weak electrolyte. conductivity is a measure of how well a solution conducts electricity. conductivity. How To Measure Ion Conductivity.
From group.chem.iastate.edu
Conductivity Meter Holme Research Group Iowa State University How To Measure Ion Conductivity As the ranges in aqueous solutions are usually small, the. conductivity meters measure the ion capacity in aqueous solution to carry electrical current. To interpret a chemical reaction by observing aqueous solution conductivity. A voltage is applied between two electrodes inside the conductivity. conductivity is measured using a conductivity meter and probe. To carry a current a solution. How To Measure Ion Conductivity.
From rosecal.com.au
How to measure electrical conductivity? Rose Calibration Company How To Measure Ion Conductivity As the ranges in aqueous solutions are usually small, the. To interpret a chemical reaction by observing aqueous solution conductivity. conductivity is a measure of how well a solution conducts electricity. conductivity is measured using a conductivity meter and probe. A voltage is applied between two electrodes inside the conductivity. the equivalent conductance increases with the charge. How To Measure Ion Conductivity.
From www.doubtnut.com
Strong acid versus strong base The principle of conductometric titr How To Measure Ion Conductivity conductivity meters measure the ion capacity in aqueous solution to carry electrical current. to determine of the solution is a strong or weak electrolyte. To carry a current a solution must contain charged. the determination of ph value, conductivity and related parameters such as ion concentration, resistivity, and salinity, are. conductivity is measured using a conductivity. How To Measure Ion Conductivity.
From matlantis.com
Evaluation Of Lithium Ion Conductivity Of Type Oxide Materials How To Measure Ion Conductivity to determine of the solution is a strong or weak electrolyte. A voltage is applied between two electrodes inside the conductivity. conductivity meters measure the ion capacity in aqueous solution to carry electrical current. To interpret a chemical reaction by observing aqueous solution conductivity. As the ranges in aqueous solutions are usually small, the. conductivity is measured. How To Measure Ion Conductivity.
From pubs.rsc.org
Local Liion conductivity changes within Al stabilized Li 7 La 3 Zr 2 O How To Measure Ion Conductivity To carry a current a solution must contain charged. To interpret a chemical reaction by observing aqueous solution conductivity. the determination of ph value, conductivity and related parameters such as ion concentration, resistivity, and salinity, are. conductivity is measured using a conductivity meter and probe. the equivalent conductance increases with the charge number and decreases with the. How To Measure Ion Conductivity.
From www.labrotovap.com
Digital Conductivity meter PH TDS EC Meter Salinity Temperature Tester How To Measure Ion Conductivity To carry a current a solution must contain charged. the determination of ph value, conductivity and related parameters such as ion concentration, resistivity, and salinity, are. conductivity meters measure the ion capacity in aqueous solution to carry electrical current. To interpret a chemical reaction by observing aqueous solution conductivity. to determine of the solution is a strong. How To Measure Ion Conductivity.
From www.slideserve.com
PPT Ionic Conductivity In A Thermochromic Solid PowerPoint How To Measure Ion Conductivity conductivity meters measure the ion capacity in aqueous solution to carry electrical current. the equivalent conductance increases with the charge number and decreases with the size. The expected values can differ from. As the ranges in aqueous solutions are usually small, the. the determination of ph value, conductivity and related parameters such as ion concentration, resistivity, and. How To Measure Ion Conductivity.
From sensorex.com
Why Electrical Conductivity of Water is Important for Industrial How To Measure Ion Conductivity to determine of the solution is a strong or weak electrolyte. conductivity is measured using a conductivity meter and probe. The expected values can differ from. conductivity is a measure of how well a solution conducts electricity. As the ranges in aqueous solutions are usually small, the. the determination of ph value, conductivity and related parameters. How To Measure Ion Conductivity.
From www.youtube.com
Class XII Chemistry Measurement of Conductivity of Ionic Solution How To Measure Ion Conductivity A voltage is applied between two electrodes inside the conductivity. To interpret a chemical reaction by observing aqueous solution conductivity. As the ranges in aqueous solutions are usually small, the. conductivity is a measure of how well a solution conducts electricity. To carry a current a solution must contain charged. the equivalent conductance increases with the charge number. How To Measure Ion Conductivity.
From em.geosci.xyz
Electrical Conductivity — Geophysics How To Measure Ion Conductivity As the ranges in aqueous solutions are usually small, the. To carry a current a solution must contain charged. The expected values can differ from. the equivalent conductance increases with the charge number and decreases with the size. conductivity is measured using a conductivity meter and probe. conductivity meters measure the ion capacity in aqueous solution to. How To Measure Ion Conductivity.
From cercell.com
Conductivity SingleUse How To Measure Ion Conductivity To carry a current a solution must contain charged. conductivity is a measure of how well a solution conducts electricity. conductivity is measured using a conductivity meter and probe. The expected values can differ from. A voltage is applied between two electrodes inside the conductivity. to determine of the solution is a strong or weak electrolyte. . How To Measure Ion Conductivity.
From www.mdpi.com
Membranes Free FullText Importance of Hydroxide Ion Conductivity How To Measure Ion Conductivity The expected values can differ from. To interpret a chemical reaction by observing aqueous solution conductivity. to determine of the solution is a strong or weak electrolyte. A voltage is applied between two electrodes inside the conductivity. To carry a current a solution must contain charged. conductivity is measured using a conductivity meter and probe. the determination. How To Measure Ion Conductivity.
From techiescientist.com
Does Ice Conduct Electricity? Techiescientist How To Measure Ion Conductivity the determination of ph value, conductivity and related parameters such as ion concentration, resistivity, and salinity, are. conductivity is a measure of how well a solution conducts electricity. conductivity is measured using a conductivity meter and probe. to determine of the solution is a strong or weak electrolyte. To carry a current a solution must contain. How To Measure Ion Conductivity.
From www.researchgate.net
How can we measure Ionic Conductivity using Impedance Spectra How To Measure Ion Conductivity To interpret a chemical reaction by observing aqueous solution conductivity. To carry a current a solution must contain charged. The expected values can differ from. As the ranges in aqueous solutions are usually small, the. to determine of the solution is a strong or weak electrolyte. conductivity is measured using a conductivity meter and probe. the determination. How To Measure Ion Conductivity.
From pubs.acs.org
How to Measure a Reliable Ionic Conductivity? The Stack Pressure How To Measure Ion Conductivity As the ranges in aqueous solutions are usually small, the. To interpret a chemical reaction by observing aqueous solution conductivity. the equivalent conductance increases with the charge number and decreases with the size. to determine of the solution is a strong or weak electrolyte. conductivity is measured using a conductivity meter and probe. To carry a current. How To Measure Ion Conductivity.
From www.researchgate.net
Ionic conductivity (a) Example of Nyquist plot. (b) Ionic conductivity How To Measure Ion Conductivity To carry a current a solution must contain charged. conductivity is a measure of how well a solution conducts electricity. conductivity is measured using a conductivity meter and probe. the equivalent conductance increases with the charge number and decreases with the size. the determination of ph value, conductivity and related parameters such as ion concentration, resistivity,. How To Measure Ion Conductivity.
From www.youtube.com
Electrical Conductivity Measurements of Thin Films YouTube How To Measure Ion Conductivity The expected values can differ from. As the ranges in aqueous solutions are usually small, the. the equivalent conductance increases with the charge number and decreases with the size. conductivity is measured using a conductivity meter and probe. To interpret a chemical reaction by observing aqueous solution conductivity. A voltage is applied between two electrodes inside the conductivity.. How To Measure Ion Conductivity.
From www.youtube.com
Measurement of the Conductivity of Ionic Solutions YouTube How To Measure Ion Conductivity the determination of ph value, conductivity and related parameters such as ion concentration, resistivity, and salinity, are. conductivity is a measure of how well a solution conducts electricity. to determine of the solution is a strong or weak electrolyte. As the ranges in aqueous solutions are usually small, the. To carry a current a solution must contain. How To Measure Ion Conductivity.
From www.slideserve.com
PPT Properties of Substances PowerPoint Presentation, free download How To Measure Ion Conductivity the determination of ph value, conductivity and related parameters such as ion concentration, resistivity, and salinity, are. conductivity is a measure of how well a solution conducts electricity. to determine of the solution is a strong or weak electrolyte. The expected values can differ from. As the ranges in aqueous solutions are usually small, the. conductivity. How To Measure Ion Conductivity.
From pilgaard.info
Conductivity Ions in solution Michael Pilgaard's Chemistry How To Measure Ion Conductivity conductivity is a measure of how well a solution conducts electricity. A voltage is applied between two electrodes inside the conductivity. to determine of the solution is a strong or weak electrolyte. To carry a current a solution must contain charged. As the ranges in aqueous solutions are usually small, the. the determination of ph value, conductivity. How To Measure Ion Conductivity.
From www.ck12.org
Physical Properties of Ionic Compounds ( Read ) Chemistry CK12 How To Measure Ion Conductivity The expected values can differ from. A voltage is applied between two electrodes inside the conductivity. the determination of ph value, conductivity and related parameters such as ion concentration, resistivity, and salinity, are. conductivity is a measure of how well a solution conducts electricity. To carry a current a solution must contain charged. conductivity is measured using. How To Measure Ion Conductivity.
From www.youtube.com
How to Calculate the activation energy from DC and AC conductivity How To Measure Ion Conductivity conductivity meters measure the ion capacity in aqueous solution to carry electrical current. A voltage is applied between two electrodes inside the conductivity. the equivalent conductance increases with the charge number and decreases with the size. to determine of the solution is a strong or weak electrolyte. The expected values can differ from. the determination of. How To Measure Ion Conductivity.
From www.researchgate.net
Ionic conductivity of OIPCs with different anions. Download How To Measure Ion Conductivity conductivity is a measure of how well a solution conducts electricity. conductivity is measured using a conductivity meter and probe. The expected values can differ from. conductivity meters measure the ion capacity in aqueous solution to carry electrical current. A voltage is applied between two electrodes inside the conductivity. to determine of the solution is a. How To Measure Ion Conductivity.
From fphoto.photoshelter.com
science chemistry conductivity test Fundamental Photographs The Art How To Measure Ion Conductivity conductivity meters measure the ion capacity in aqueous solution to carry electrical current. to determine of the solution is a strong or weak electrolyte. conductivity is measured using a conductivity meter and probe. conductivity is a measure of how well a solution conducts electricity. the equivalent conductance increases with the charge number and decreases with. How To Measure Ion Conductivity.
From www.researchgate.net
Ionic conductivity calculated from chemical diffusivity measurements on How To Measure Ion Conductivity to determine of the solution is a strong or weak electrolyte. The expected values can differ from. To interpret a chemical reaction by observing aqueous solution conductivity. conductivity meters measure the ion capacity in aqueous solution to carry electrical current. A voltage is applied between two electrodes inside the conductivity. the determination of ph value, conductivity and. How To Measure Ion Conductivity.
From blog.bluelab.com
What is conductivity and how do you measure it? How To Measure Ion Conductivity conductivity is a measure of how well a solution conducts electricity. conductivity is measured using a conductivity meter and probe. As the ranges in aqueous solutions are usually small, the. the equivalent conductance increases with the charge number and decreases with the size. The expected values can differ from. A voltage is applied between two electrodes inside. How To Measure Ion Conductivity.
From control.com
Measuring Electrical Conductivity Introduction to Continuous How To Measure Ion Conductivity As the ranges in aqueous solutions are usually small, the. To carry a current a solution must contain charged. the equivalent conductance increases with the charge number and decreases with the size. To interpret a chemical reaction by observing aqueous solution conductivity. to determine of the solution is a strong or weak electrolyte. conductivity is measured using. How To Measure Ion Conductivity.
From www.science.org
Enhancing ionic conductivity in solid electrolyte by relocating How To Measure Ion Conductivity A voltage is applied between two electrodes inside the conductivity. The expected values can differ from. As the ranges in aqueous solutions are usually small, the. to determine of the solution is a strong or weak electrolyte. To carry a current a solution must contain charged. To interpret a chemical reaction by observing aqueous solution conductivity. the equivalent. How To Measure Ion Conductivity.
From www.researchgate.net
Ionic conductivity of proteinbased ion conductor (PIC) at room How To Measure Ion Conductivity conductivity is a measure of how well a solution conducts electricity. conductivity is measured using a conductivity meter and probe. to determine of the solution is a strong or weak electrolyte. A voltage is applied between two electrodes inside the conductivity. To carry a current a solution must contain charged. the equivalent conductance increases with the. How To Measure Ion Conductivity.
From atlas-scientific.com
How Do Ions Increase Conductivity? Atlas Scientific How To Measure Ion Conductivity conductivity is a measure of how well a solution conducts electricity. As the ranges in aqueous solutions are usually small, the. A voltage is applied between two electrodes inside the conductivity. conductivity meters measure the ion capacity in aqueous solution to carry electrical current. the determination of ph value, conductivity and related parameters such as ion concentration,. How To Measure Ion Conductivity.