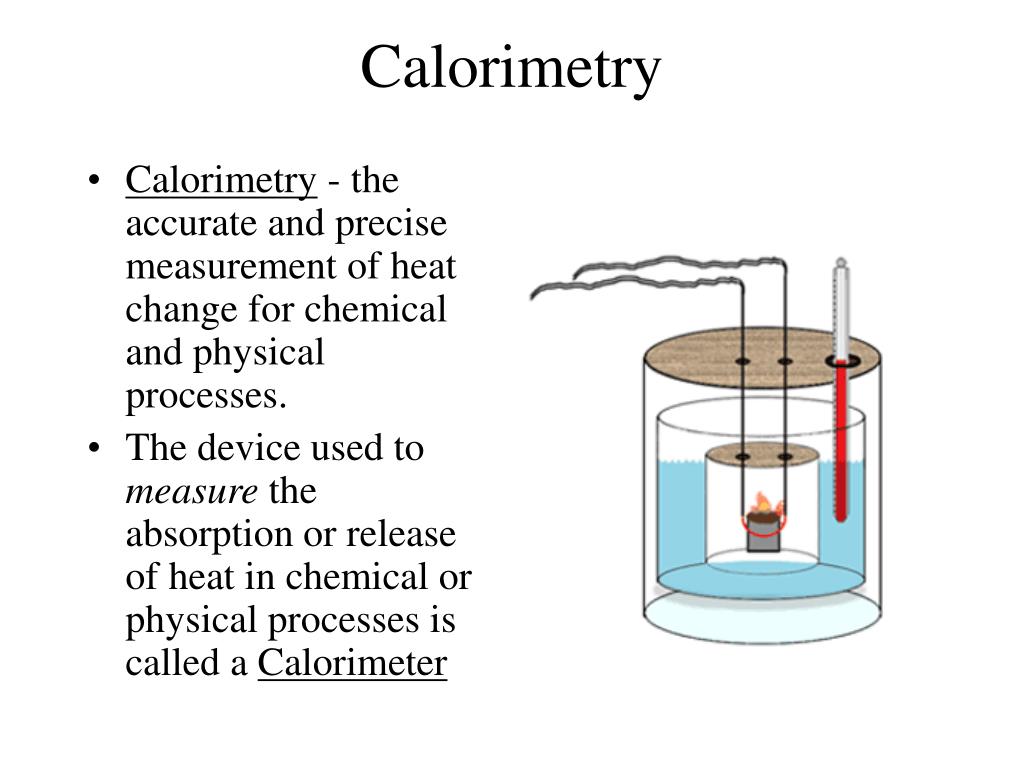
Negative Calorimeter Constant Meaning . a negative δh means that heat flows from a system to its surroundings; we note the heat transferred from the reaction is abosorbed by the calorimeter and its contents, noting that the calorimeter. figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal. A positive δh means that heat flows into a system from. the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. If the constant were zero, the final temperature of the 50.0 g of water would be 42.5 °c. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. 1) since we know the real. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from.
from www.slideserve.com
in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from. If the constant were zero, the final temperature of the 50.0 g of water would be 42.5 °c. figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal. we note the heat transferred from the reaction is abosorbed by the calorimeter and its contents, noting that the calorimeter. 1) since we know the real. A positive δh means that heat flows into a system from. a negative δh means that heat flows from a system to its surroundings; the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow.
PPT Chapter 11 PowerPoint Presentation, free download ID1084959
Negative Calorimeter Constant Meaning the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal. If the constant were zero, the final temperature of the 50.0 g of water would be 42.5 °c. the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from. we note the heat transferred from the reaction is abosorbed by the calorimeter and its contents, noting that the calorimeter. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. 1) since we know the real. a negative δh means that heat flows from a system to its surroundings; A positive δh means that heat flows into a system from.
From www.chegg.com
Solved Use the Calorimeter Constant and equations provided Negative Calorimeter Constant Meaning figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal. the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. 1) since we know the real. a negative δh means that heat flows from a system to its surroundings;. Negative Calorimeter Constant Meaning.
From www.chegg.com
Chemistry Archive November 16, 2016 Negative Calorimeter Constant Meaning in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. If the constant were zero, the final temperature of the 50.0 g of water would be 42.5 °c. we. Negative Calorimeter Constant Meaning.
From www.numerade.com
SOLVED Calorimetry Lab 1. In the measurement of a calorimeter Negative Calorimeter Constant Meaning A positive δh means that heat flows into a system from. a negative δh means that heat flows from a system to its surroundings; we note the heat transferred from the reaction is abosorbed by the calorimeter and its contents, noting that the calorimeter. the calorimeters described are designed to operate at constant (atmospheric) pressure and are. Negative Calorimeter Constant Meaning.
From www.youtube.com
3 problems of specific heat & calorimeter (1st year secondary second Negative Calorimeter Constant Meaning in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. we note the heat transferred from the reaction is abosorbed by the calorimeter and its contents, noting that the calorimeter. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that. Negative Calorimeter Constant Meaning.
From www.chegg.com
Calculate the calorimeter constant (From Lab) So for Negative Calorimeter Constant Meaning figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. we note the heat transferred from the reaction is abosorbed by the calorimeter and its contents, noting. Negative Calorimeter Constant Meaning.
From saylordotorg.github.io
Calorimetry Negative Calorimeter Constant Meaning A positive δh means that heat flows into a system from. figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal. a negative δh means that heat flows from a system to its surroundings; in a calorimetric determination, either (a) an exothermic process occurs and heat, q,. Negative Calorimeter Constant Meaning.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Negative Calorimeter Constant Meaning figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal. A positive δh means that heat flows into a system from. the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. If the constant were zero, the final temperature of. Negative Calorimeter Constant Meaning.
From courses.lumenlearning.com
Calorimetry General Chemistry Negative Calorimeter Constant Meaning we note the heat transferred from the reaction is abosorbed by the calorimeter and its contents, noting that the calorimeter. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. 1) since we know the real. the calorimeters described are designed to operate at constant (atmospheric) pressure. Negative Calorimeter Constant Meaning.
From www.youtube.com
How to find calorimeter constant YouTube Negative Calorimeter Constant Meaning the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. If the constant were zero, the final temperature of the 50.0 g of water would be 42.5 °c. we note the heat transferred from the reaction is abosorbed by the calorimeter and its contents, noting that the calorimeter. a. Negative Calorimeter Constant Meaning.
From www.reddit.com
Calculating calorimeter constant is coming out negative and don’t know Negative Calorimeter Constant Meaning If the constant were zero, the final temperature of the 50.0 g of water would be 42.5 °c. figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is.. Negative Calorimeter Constant Meaning.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Negative Calorimeter Constant Meaning A positive δh means that heat flows into a system from. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. we note the heat transferred from the reaction is abosorbed by the calorimeter and its contents, noting that the calorimeter. figure 5.11 in a calorimetric determination,. Negative Calorimeter Constant Meaning.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Negative Calorimeter Constant Meaning we note the heat transferred from the reaction is abosorbed by the calorimeter and its contents, noting that the calorimeter. figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that. Negative Calorimeter Constant Meaning.
From app.jove.com
Constant Pressure Calorimeter/ Coffee Cup Calorimeter Concept Negative Calorimeter Constant Meaning figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal. the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy. Negative Calorimeter Constant Meaning.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Negative Calorimeter Constant Meaning figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal. a negative δh means that heat flows from a system to its surroundings; the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. in a calorimetric determination, either. Negative Calorimeter Constant Meaning.
From www.slideserve.com
PPT Zumdahl Chapter 6 PowerPoint Presentation, free download ID4269919 Negative Calorimeter Constant Meaning in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q,. Negative Calorimeter Constant Meaning.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Negative Calorimeter Constant Meaning a negative δh means that heat flows from a system to its surroundings; 1) since we know the real. A positive δh means that heat flows into a system from. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from. If the constant were zero, the. Negative Calorimeter Constant Meaning.
From www.chegg.com
Solved The Model Bomb Calorimetry Motorized stirrer Negative Calorimeter Constant Meaning figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from. A positive δh means that heat flows into a system from. 1) since we know the. Negative Calorimeter Constant Meaning.
From courses.lumenlearning.com
Calorimetry Chemistry I Negative Calorimeter Constant Meaning A positive δh means that heat flows into a system from. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal. we note the heat transferred from. Negative Calorimeter Constant Meaning.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Negative Calorimeter Constant Meaning A positive δh means that heat flows into a system from. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. a negative δh means that heat flows from a system to its surroundings; If the constant were zero, the final temperature of the 50.0 g of water. Negative Calorimeter Constant Meaning.
From www.youtube.com
Calorimetry at Constant Pressure YouTube Negative Calorimeter Constant Meaning we note the heat transferred from the reaction is abosorbed by the calorimeter and its contents, noting that the calorimeter. figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal. the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat. Negative Calorimeter Constant Meaning.
From www.youtube.com
Calorimeter Constant YouTube Negative Calorimeter Constant Meaning in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. a negative δh means that heat flows from a system to its surroundings; in a calorimetric determination, either. Negative Calorimeter Constant Meaning.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Negative Calorimeter Constant Meaning in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that. Negative Calorimeter Constant Meaning.
From physics.stackexchange.com
temperature Thermodynamics Units in calculations of heat (Q Negative Calorimeter Constant Meaning figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal. If the constant were zero, the final temperature of the 50.0 g of water would be 42.5 °c. 1) since we know the real. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is. Negative Calorimeter Constant Meaning.
From www.thoughtco.com
Calorimeter Definition in Chemistry Negative Calorimeter Constant Meaning we note the heat transferred from the reaction is abosorbed by the calorimeter and its contents, noting that the calorimeter. figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that. Negative Calorimeter Constant Meaning.
From graphingstyle.blogspot.com
View Constant Term Calculator The Latest Graphing Negative Calorimeter Constant Meaning in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from. A positive δh means that heat flows into a system from. If the constant were zero, the final temperature of the 50.0 g of water would be 42.5 °c. a negative δh means that heat flows. Negative Calorimeter Constant Meaning.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Negative Calorimeter Constant Meaning in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from. a negative δh means that heat flows from a system to its surroundings; If the constant were zero, the final temperature of the 50.0 g of water would be 42.5 °c. figure 5.11 in a. Negative Calorimeter Constant Meaning.
From www.youtube.com
Principle of Calorimetry YouTube Negative Calorimeter Constant Meaning in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. we note the heat transferred from the reaction is abosorbed by the calorimeter and its contents, noting that the calorimeter. A positive δh means that heat flows into a system from. 1) since we know the real. . Negative Calorimeter Constant Meaning.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow Negative Calorimeter Constant Meaning A positive δh means that heat flows into a system from. figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal. the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. in a calorimetric determination, either (a) an exothermic. Negative Calorimeter Constant Meaning.
From www.youtube.com
Calorimetry calculation YouTube Negative Calorimeter Constant Meaning If the constant were zero, the final temperature of the 50.0 g of water would be 42.5 °c. the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. we note the heat transferred from the reaction is abosorbed by the calorimeter and its contents, noting that the calorimeter. A positive. Negative Calorimeter Constant Meaning.
From courses.lumenlearning.com
Calorimetry Introductory Chemistry Lecture & Lab Negative Calorimeter Constant Meaning figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from. we note the heat transferred from the reaction is abosorbed by the calorimeter and its. Negative Calorimeter Constant Meaning.
From haipernews.com
How To Calculate Heat Capacity From Calorimeter Haiper Negative Calorimeter Constant Meaning A positive δh means that heat flows into a system from. If the constant were zero, the final temperature of the 50.0 g of water would be 42.5 °c. a negative δh means that heat flows from a system to its surroundings; in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating. Negative Calorimeter Constant Meaning.
From socratic.org
A student conducting a calorimetry investigation determines a negative Negative Calorimeter Constant Meaning figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal. the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy. Negative Calorimeter Constant Meaning.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2692866 Negative Calorimeter Constant Meaning a negative δh means that heat flows from a system to its surroundings; the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. we note the heat transferred from the reaction is abosorbed by the calorimeter and its contents, noting that the calorimeter. figure 5.11 in a calorimetric. Negative Calorimeter Constant Meaning.
From www.youtube.com
Calorimeter Constant lab part 1 YouTube Negative Calorimeter Constant Meaning in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. 1) since we know the real. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from. the calorimeters described are designed to operate at constant. Negative Calorimeter Constant Meaning.
From www.youtube.com
050 Calorimetry YouTube Negative Calorimeter Constant Meaning figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal. If the constant were zero, the final temperature of the 50.0 g of water would be 42.5 °c. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. Negative Calorimeter Constant Meaning.