Titration Curve Of Hcl And Naoh . a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is. learn how to use titration curves to determine the acid dissociation constant (ka) of a weak acid. As we add strong base to a strong acid, the ph increases. a summary of the important curves. The way you normally carry out a titration involves adding the acid to the alkali. — learn how to determine hydrochloric acid concentration by titrating it with sodium hydroxide. See examples of titration curves for strong and weak acids, and how. — the ph curve diagram below represents the titration of a strong acid with a strong base: — complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml of 0.288 m hcl with. Find reaction, end point detection, solutions, procedure,.
from mungfali.com
— learn how to determine hydrochloric acid concentration by titrating it with sodium hydroxide. a summary of the important curves. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is. learn how to use titration curves to determine the acid dissociation constant (ka) of a weak acid. As we add strong base to a strong acid, the ph increases. — complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml of 0.288 m hcl with. The way you normally carry out a titration involves adding the acid to the alkali. — the ph curve diagram below represents the titration of a strong acid with a strong base: Find reaction, end point detection, solutions, procedure,. See examples of titration curves for strong and weak acids, and how.
HCl NaOH Titration
Titration Curve Of Hcl And Naoh As we add strong base to a strong acid, the ph increases. The way you normally carry out a titration involves adding the acid to the alkali. As we add strong base to a strong acid, the ph increases. See examples of titration curves for strong and weak acids, and how. — learn how to determine hydrochloric acid concentration by titrating it with sodium hydroxide. a summary of the important curves. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is. Find reaction, end point detection, solutions, procedure,. — complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml of 0.288 m hcl with. — the ph curve diagram below represents the titration of a strong acid with a strong base: learn how to use titration curves to determine the acid dissociation constant (ka) of a weak acid.
From byjus.com
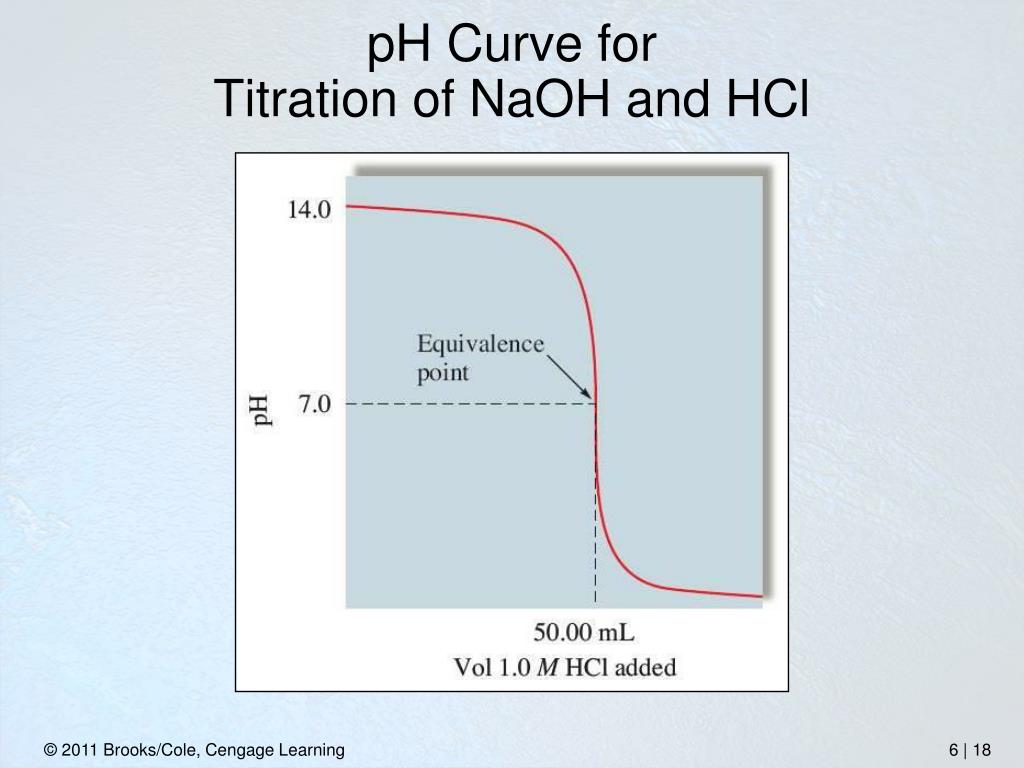
The graph of pH during the titration of NaOH and HCl Titration Curve Of Hcl And Naoh As we add strong base to a strong acid, the ph increases. Find reaction, end point detection, solutions, procedure,. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is. See examples of titration curves for strong and weak acids, and how. —. Titration Curve Of Hcl And Naoh.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID5723241 Titration Curve Of Hcl And Naoh a summary of the important curves. See examples of titration curves for strong and weak acids, and how. As we add strong base to a strong acid, the ph increases. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is. learn. Titration Curve Of Hcl And Naoh.
From www.researchgate.net
Potentiometric titration curves of HCl with NaOH using the AISI 304... Download Scientific Diagram Titration Curve Of Hcl And Naoh — complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml of 0.288 m hcl with. Find reaction, end point detection, solutions, procedure,. — learn how to determine hydrochloric acid concentration by titrating it with sodium hydroxide. See examples of titration curves for strong and. Titration Curve Of Hcl And Naoh.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration Curve Of Hcl And Naoh learn how to use titration curves to determine the acid dissociation constant (ka) of a weak acid. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is. See examples of titration curves for strong and weak acids, and how. a summary. Titration Curve Of Hcl And Naoh.
From www.youtube.com
Titration of a monoprotic strong acid (HCl) and monoprotic strong base (NaOH) YouTube Titration Curve Of Hcl And Naoh a summary of the important curves. learn how to use titration curves to determine the acid dissociation constant (ka) of a weak acid. — learn how to determine hydrochloric acid concentration by titrating it with sodium hydroxide. See examples of titration curves for strong and weak acids, and how. a titration is carried out for 25.00. Titration Curve Of Hcl And Naoh.
From exomuvqjn.blob.core.windows.net
What Happens When You Mix Hcl And Naoh at Christopher Schiller blog Titration Curve Of Hcl And Naoh a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is. learn how to use titration curves to determine the acid dissociation constant (ka) of a weak acid. See examples of titration curves for strong and weak acids, and how. — the. Titration Curve Of Hcl And Naoh.
From exogrnoza.blob.core.windows.net
Titration Curve Acetic Acid And Naoh at Isabel Keith blog Titration Curve Of Hcl And Naoh a summary of the important curves. — complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml of 0.288 m hcl with. — learn how to determine hydrochloric acid concentration by titrating it with sodium hydroxide. Find reaction, end point detection, solutions, procedure,. . Titration Curve Of Hcl And Naoh.
From pubs.sciepub.com
Figure 5B. Plot of the titration of strong acid (HCl= 0.1M) with strong base (NaOH=0.1M Titration Curve Of Hcl And Naoh — learn how to determine hydrochloric acid concentration by titrating it with sodium hydroxide. Find reaction, end point detection, solutions, procedure,. learn how to use titration curves to determine the acid dissociation constant (ka) of a weak acid. — complete the following table and generate a titration curve showing the ph versus volume of added base for. Titration Curve Of Hcl And Naoh.
From www.researchgate.net
4. Hydrochloric acid and sodium hydroxide neutralisation curves, [99] Download Scientific Diagram Titration Curve Of Hcl And Naoh a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is. See examples of titration curves for strong and weak acids, and how. a summary of the important curves. The way you normally carry out a titration involves adding the acid to the. Titration Curve Of Hcl And Naoh.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Titration Curve Of Hcl And Naoh a summary of the important curves. Find reaction, end point detection, solutions, procedure,. See examples of titration curves for strong and weak acids, and how. — complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml of 0.288 m hcl with. learn how to. Titration Curve Of Hcl And Naoh.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Titration Curve Of Hcl And Naoh See examples of titration curves for strong and weak acids, and how. — learn how to determine hydrochloric acid concentration by titrating it with sodium hydroxide. a summary of the important curves. learn how to use titration curves to determine the acid dissociation constant (ka) of a weak acid. — complete the following table and generate. Titration Curve Of Hcl And Naoh.
From www.researchgate.net
Conductometric titration of hydrochloric acid 0.1 M with sodium... Download Scientific Diagram Titration Curve Of Hcl And Naoh learn how to use titration curves to determine the acid dissociation constant (ka) of a weak acid. — complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml of 0.288 m hcl with. — the ph curve diagram below represents the titration of a. Titration Curve Of Hcl And Naoh.
From www.chemistryscl.com
NaOH and HCl Titration Curves Selecting Indicators Titration Curve Of Hcl And Naoh a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is. As we add strong base to a strong acid, the ph increases. — learn how to determine hydrochloric acid concentration by titrating it with sodium hydroxide. learn how to use titration. Titration Curve Of Hcl And Naoh.
From www.chemistryscl.com
NaOH and HCl Titration Curves Selecting Indicators Titration Curve Of Hcl And Naoh The way you normally carry out a titration involves adding the acid to the alkali. — learn how to determine hydrochloric acid concentration by titrating it with sodium hydroxide. — complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml of 0.288 m hcl with.. Titration Curve Of Hcl And Naoh.
From www.chegg.com
Solved Use the following titration curve for the first four Titration Curve Of Hcl And Naoh — complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml of 0.288 m hcl with. Find reaction, end point detection, solutions, procedure,. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh. Titration Curve Of Hcl And Naoh.
From dxoqraial.blob.core.windows.net
Titration Reaction Of Hcl And Naoh at Lucia Chamberlain blog Titration Curve Of Hcl And Naoh As we add strong base to a strong acid, the ph increases. learn how to use titration curves to determine the acid dissociation constant (ka) of a weak acid. — learn how to determine hydrochloric acid concentration by titrating it with sodium hydroxide. — the ph curve diagram below represents the titration of a strong acid with. Titration Curve Of Hcl And Naoh.
From schoolbag.info
Titration and Buffers Acids and Bases Training MCAT General Chemistry Review Titration Curve Of Hcl And Naoh a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is. See examples of titration curves for strong and weak acids, and how. The way you normally carry out a titration involves adding the acid to the alkali. a summary of the important. Titration Curve Of Hcl And Naoh.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration Curve Of Hcl And Naoh — complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml of 0.288 m hcl with. learn how to use titration curves to determine the acid dissociation constant (ka) of a weak acid. — the ph curve diagram below represents the titration of a. Titration Curve Of Hcl And Naoh.
From mungfali.com
Titration Graph Titration Curve Of Hcl And Naoh As we add strong base to a strong acid, the ph increases. learn how to use titration curves to determine the acid dissociation constant (ka) of a weak acid. — the ph curve diagram below represents the titration of a strong acid with a strong base: — learn how to determine hydrochloric acid concentration by titrating it. Titration Curve Of Hcl And Naoh.
From www.researchgate.net
Titration curves of hydrochloricacidbased electrograining solution... Download Scientific Titration Curve Of Hcl And Naoh — learn how to determine hydrochloric acid concentration by titrating it with sodium hydroxide. — the ph curve diagram below represents the titration of a strong acid with a strong base: a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is.. Titration Curve Of Hcl And Naoh.
From mungfali.com
HCl NaOH Titration Titration Curve Of Hcl And Naoh — learn how to determine hydrochloric acid concentration by titrating it with sodium hydroxide. Find reaction, end point detection, solutions, procedure,. — complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml of 0.288 m hcl with. — the ph curve diagram below represents. Titration Curve Of Hcl And Naoh.
From exoyzonai.blob.core.windows.net
Titration Curve Labeled Buffer Region at Craig Johnson blog Titration Curve Of Hcl And Naoh — complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml of 0.288 m hcl with. learn how to use titration curves to determine the acid dissociation constant (ka) of a weak acid. — the ph curve diagram below represents the titration of a. Titration Curve Of Hcl And Naoh.
From studylib.net
Titration of HCl with NaOH Titration Curve Of Hcl And Naoh — complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml of 0.288 m hcl with. The way you normally carry out a titration involves adding the acid to the alkali. — the ph curve diagram below represents the titration of a strong acid with. Titration Curve Of Hcl And Naoh.
From mungfali.com
HCl NaOH Titration Titration Curve Of Hcl And Naoh — learn how to determine hydrochloric acid concentration by titrating it with sodium hydroxide. — complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml of 0.288 m hcl with. The way you normally carry out a titration involves adding the acid to the alkali.. Titration Curve Of Hcl And Naoh.
From www.chegg.com
Solved HCl NaOH Titration CurveCH3COOH NaOH Titration Titration Curve Of Hcl And Naoh The way you normally carry out a titration involves adding the acid to the alkali. — complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml of 0.288 m hcl with. a summary of the important curves. a titration is carried out for 25.00. Titration Curve Of Hcl And Naoh.
From mungfali.com
HCl NaOH Titration Titration Curve Of Hcl And Naoh The way you normally carry out a titration involves adding the acid to the alkali. — complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml of 0.288 m hcl with. As we add strong base to a strong acid, the ph increases. learn how. Titration Curve Of Hcl And Naoh.
From mariela-kcarroll.blogspot.com
Titration Curve of Hcl and Naoh Titration Curve Of Hcl And Naoh See examples of titration curves for strong and weak acids, and how. — complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml of 0.288 m hcl with. The way you normally carry out a titration involves adding the acid to the alkali. a titration. Titration Curve Of Hcl And Naoh.
From tukioka-clinic.com
😂 Titration of naoh and na2co3 with hcl. Titration Of Hcl And Na2co3. 20190211 Titration Curve Of Hcl And Naoh a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is. learn how to use titration curves to determine the acid dissociation constant (ka) of a weak acid. Find reaction, end point detection, solutions, procedure,. The way you normally carry out a titration. Titration Curve Of Hcl And Naoh.
From www.numerade.com
SOLVED 4) (4 pts) In lab you titrated HCl with NaOH Sketch the titration curve (pH versus Titration Curve Of Hcl And Naoh — learn how to determine hydrochloric acid concentration by titrating it with sodium hydroxide. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is. — complete the following table and generate a titration curve showing the ph versus volume of added. Titration Curve Of Hcl And Naoh.
From www.youtube.com
Conductometric titration I strong acid (HCl) versus strong base titration (NaOH) YouTube Titration Curve Of Hcl And Naoh As we add strong base to a strong acid, the ph increases. Find reaction, end point detection, solutions, procedure,. The way you normally carry out a titration involves adding the acid to the alkali. — the ph curve diagram below represents the titration of a strong acid with a strong base: a summary of the important curves. . Titration Curve Of Hcl And Naoh.
From dxoqraial.blob.core.windows.net
Titration Reaction Of Hcl And Naoh at Lucia Chamberlain blog Titration Curve Of Hcl And Naoh a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is. Find reaction, end point detection, solutions, procedure,. — learn how to determine hydrochloric acid concentration by titrating it with sodium hydroxide. learn how to use titration curves to determine the acid. Titration Curve Of Hcl And Naoh.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Curve Of Hcl And Naoh a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is. See examples of titration curves for strong and weak acids, and how. As we add strong base to a strong acid, the ph increases. — complete the following table and generate a. Titration Curve Of Hcl And Naoh.
From www.chemistryscl.com
AcidBase Titration, Indicators and pH Curves Titration Curve Of Hcl And Naoh The way you normally carry out a titration involves adding the acid to the alkali. As we add strong base to a strong acid, the ph increases. — learn how to determine hydrochloric acid concentration by titrating it with sodium hydroxide. — the ph curve diagram below represents the titration of a strong acid with a strong base:. Titration Curve Of Hcl And Naoh.
From chart-studio.plotly.com
KHP and NaOH Titration Curve line chart made by Kylclk plotly Titration Curve Of Hcl And Naoh See examples of titration curves for strong and weak acids, and how. As we add strong base to a strong acid, the ph increases. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is. learn how to use titration curves to determine. Titration Curve Of Hcl And Naoh.
From www.sarthaks.com
conductometric titration curve of a equimolar mixture of a HCl and HCN with NaOH(aq) is Titration Curve Of Hcl And Naoh — complete the following table and generate a titration curve showing the ph versus volume of added base for the titration of 50.0 ml of 0.288 m hcl with. — the ph curve diagram below represents the titration of a strong acid with a strong base: — learn how to determine hydrochloric acid concentration by titrating it. Titration Curve Of Hcl And Naoh.