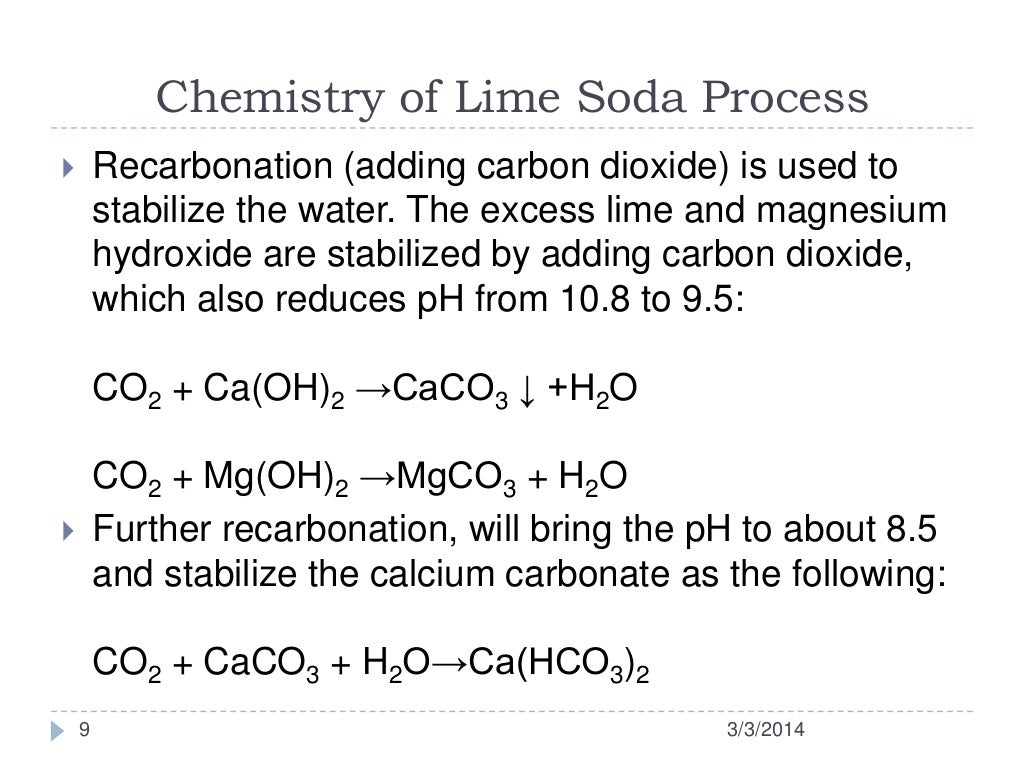
How Does Lime Softening Work . A combination of lime and soda ash, along with coagulant and flocculant chemicals, is added to raw water to promote a precipitation reaction. Learn about lime softening in this excerpt from the lime softening lecture from our water treatment exam prep course visit our website. The high ph associated with lime softening can aid in. Soda ash is used to remove chemicals that cause. As in the chlorination process, lime reacts with substances in the water before it can begin softening the water. Softening often removes iron and manganese, reduces tastes and odors, reduces total solids content, and removes radioactivity. Lime is used to remove chemicals that cause carbonate hardness. This allows softening to take place.
from www.slideshare.net
As in the chlorination process, lime reacts with substances in the water before it can begin softening the water. Lime is used to remove chemicals that cause carbonate hardness. Soda ash is used to remove chemicals that cause. Softening often removes iron and manganese, reduces tastes and odors, reduces total solids content, and removes radioactivity. A combination of lime and soda ash, along with coagulant and flocculant chemicals, is added to raw water to promote a precipitation reaction. Learn about lime softening in this excerpt from the lime softening lecture from our water treatment exam prep course visit our website. This allows softening to take place. The high ph associated with lime softening can aid in.
Lime Soda Process Softening Of Water
How Does Lime Softening Work As in the chlorination process, lime reacts with substances in the water before it can begin softening the water. This allows softening to take place. Soda ash is used to remove chemicals that cause. Lime is used to remove chemicals that cause carbonate hardness. The high ph associated with lime softening can aid in. As in the chlorination process, lime reacts with substances in the water before it can begin softening the water. A combination of lime and soda ash, along with coagulant and flocculant chemicals, is added to raw water to promote a precipitation reaction. Learn about lime softening in this excerpt from the lime softening lecture from our water treatment exam prep course visit our website. Softening often removes iron and manganese, reduces tastes and odors, reduces total solids content, and removes radioactivity.
From www.slideserve.com
PPT Effective Lime/Soda Ash Water Softening PowerPoint Presentation How Does Lime Softening Work The high ph associated with lime softening can aid in. Learn about lime softening in this excerpt from the lime softening lecture from our water treatment exam prep course visit our website. A combination of lime and soda ash, along with coagulant and flocculant chemicals, is added to raw water to promote a precipitation reaction. Lime is used to remove. How Does Lime Softening Work.
From www.slideserve.com
PPT Treatment of Waste Brine from a Brackish Reverse Osmosis Plant How Does Lime Softening Work Soda ash is used to remove chemicals that cause. A combination of lime and soda ash, along with coagulant and flocculant chemicals, is added to raw water to promote a precipitation reaction. Softening often removes iron and manganese, reduces tastes and odors, reduces total solids content, and removes radioactivity. Learn about lime softening in this excerpt from the lime softening. How Does Lime Softening Work.
From bdtextileinfo.blogspot.com
Water softening process Lime soda process Bdtextileblog Textile How Does Lime Softening Work As in the chlorination process, lime reacts with substances in the water before it can begin softening the water. Lime is used to remove chemicals that cause carbonate hardness. This allows softening to take place. Learn about lime softening in this excerpt from the lime softening lecture from our water treatment exam prep course visit our website. Soda ash is. How Does Lime Softening Work.
From www.youtube.com
Lime Soda water softening process Easy way to remember reactions How Does Lime Softening Work Soda ash is used to remove chemicals that cause. Lime is used to remove chemicals that cause carbonate hardness. The high ph associated with lime softening can aid in. This allows softening to take place. Softening often removes iron and manganese, reduces tastes and odors, reduces total solids content, and removes radioactivity. A combination of lime and soda ash, along. How Does Lime Softening Work.
From www.youtube.com
Lime soda Process of Water Softening _Water Treatment_by Dr. Rekha Nair How Does Lime Softening Work As in the chlorination process, lime reacts with substances in the water before it can begin softening the water. Learn about lime softening in this excerpt from the lime softening lecture from our water treatment exam prep course visit our website. This allows softening to take place. Lime is used to remove chemicals that cause carbonate hardness. The high ph. How Does Lime Softening Work.
From www.youtube.com
Softening reactor, lime [en] YouTube How Does Lime Softening Work A combination of lime and soda ash, along with coagulant and flocculant chemicals, is added to raw water to promote a precipitation reaction. Soda ash is used to remove chemicals that cause. Softening often removes iron and manganese, reduces tastes and odors, reduces total solids content, and removes radioactivity. Learn about lime softening in this excerpt from the lime softening. How Does Lime Softening Work.
From www.youtube.com
Techniques For water Softening Hot Lime Soda Process By Dr. Nisha How Does Lime Softening Work The high ph associated with lime softening can aid in. Lime is used to remove chemicals that cause carbonate hardness. This allows softening to take place. A combination of lime and soda ash, along with coagulant and flocculant chemicals, is added to raw water to promote a precipitation reaction. Learn about lime softening in this excerpt from the lime softening. How Does Lime Softening Work.
From www.slideshare.net
Lime Soda Process Softening Of Water How Does Lime Softening Work As in the chlorination process, lime reacts with substances in the water before it can begin softening the water. Soda ash is used to remove chemicals that cause. A combination of lime and soda ash, along with coagulant and flocculant chemicals, is added to raw water to promote a precipitation reaction. Lime is used to remove chemicals that cause carbonate. How Does Lime Softening Work.
From www.slideserve.com
PPT Water Softening by Precipitation with Lime at the Bloomington How Does Lime Softening Work This allows softening to take place. The high ph associated with lime softening can aid in. A combination of lime and soda ash, along with coagulant and flocculant chemicals, is added to raw water to promote a precipitation reaction. Lime is used to remove chemicals that cause carbonate hardness. Soda ash is used to remove chemicals that cause. Learn about. How Does Lime Softening Work.
From www.youtube.com
Lime Soda Process Hot Lime Soda Process Cold Lime Soda Process How Does Lime Softening Work Lime is used to remove chemicals that cause carbonate hardness. A combination of lime and soda ash, along with coagulant and flocculant chemicals, is added to raw water to promote a precipitation reaction. The high ph associated with lime softening can aid in. Learn about lime softening in this excerpt from the lime softening lecture from our water treatment exam. How Does Lime Softening Work.
From graver.com
Lime Softeners Graver Water Systems How Does Lime Softening Work Lime is used to remove chemicals that cause carbonate hardness. Soda ash is used to remove chemicals that cause. Learn about lime softening in this excerpt from the lime softening lecture from our water treatment exam prep course visit our website. Softening often removes iron and manganese, reduces tastes and odors, reduces total solids content, and removes radioactivity. A combination. How Does Lime Softening Work.
From www.youtube.com
LimeSoda Process for water softening Types of LimeSoda Process How Does Lime Softening Work Learn about lime softening in this excerpt from the lime softening lecture from our water treatment exam prep course visit our website. Softening often removes iron and manganese, reduces tastes and odors, reduces total solids content, and removes radioactivity. Soda ash is used to remove chemicals that cause. A combination of lime and soda ash, along with coagulant and flocculant. How Does Lime Softening Work.
From thehomesteadguide.com
How Do Water Softeners Work? Simple StepByStep Process Explained How Does Lime Softening Work A combination of lime and soda ash, along with coagulant and flocculant chemicals, is added to raw water to promote a precipitation reaction. Soda ash is used to remove chemicals that cause. Softening often removes iron and manganese, reduces tastes and odors, reduces total solids content, and removes radioactivity. As in the chlorination process, lime reacts with substances in the. How Does Lime Softening Work.
From www.youtube.com
Lime Softening YouTube How Does Lime Softening Work The high ph associated with lime softening can aid in. Learn about lime softening in this excerpt from the lime softening lecture from our water treatment exam prep course visit our website. As in the chlorination process, lime reacts with substances in the water before it can begin softening the water. Soda ash is used to remove chemicals that cause.. How Does Lime Softening Work.
From www.slideshare.net
Lime Soda Process Softening Of Water How Does Lime Softening Work Softening often removes iron and manganese, reduces tastes and odors, reduces total solids content, and removes radioactivity. This allows softening to take place. Soda ash is used to remove chemicals that cause. Lime is used to remove chemicals that cause carbonate hardness. The high ph associated with lime softening can aid in. As in the chlorination process, lime reacts with. How Does Lime Softening Work.
From www.youtube.com
Cold Lime Softening Solids Removal with Flocculation & Flotation YouTube How Does Lime Softening Work Lime is used to remove chemicals that cause carbonate hardness. Soda ash is used to remove chemicals that cause. A combination of lime and soda ash, along with coagulant and flocculant chemicals, is added to raw water to promote a precipitation reaction. This allows softening to take place. The high ph associated with lime softening can aid in. Softening often. How Does Lime Softening Work.
From dynamixinc.com
Key Factors to Lime Slurry Usage in Water Treatment Processes How Does Lime Softening Work The high ph associated with lime softening can aid in. A combination of lime and soda ash, along with coagulant and flocculant chemicals, is added to raw water to promote a precipitation reaction. As in the chlorination process, lime reacts with substances in the water before it can begin softening the water. This allows softening to take place. Learn about. How Does Lime Softening Work.
From www.youtube.com
ENE 483 Lime Softening Process Objectives and Softening Reactions How Does Lime Softening Work Learn about lime softening in this excerpt from the lime softening lecture from our water treatment exam prep course visit our website. The high ph associated with lime softening can aid in. This allows softening to take place. As in the chlorination process, lime reacts with substances in the water before it can begin softening the water. A combination of. How Does Lime Softening Work.
From water.mecc.edu
Lime Soda Ash Softening How Does Lime Softening Work Lime is used to remove chemicals that cause carbonate hardness. Learn about lime softening in this excerpt from the lime softening lecture from our water treatment exam prep course visit our website. As in the chlorination process, lime reacts with substances in the water before it can begin softening the water. Softening often removes iron and manganese, reduces tastes and. How Does Lime Softening Work.
From www.slideserve.com
PPT Lime Softening PowerPoint Presentation, free download ID2529217 How Does Lime Softening Work This allows softening to take place. The high ph associated with lime softening can aid in. As in the chlorination process, lime reacts with substances in the water before it can begin softening the water. A combination of lime and soda ash, along with coagulant and flocculant chemicals, is added to raw water to promote a precipitation reaction. Soda ash. How Does Lime Softening Work.
From contendo.ca
Hot Lime Softener (HLS) Operation SAGD Oil Sands Online Training How Does Lime Softening Work Soda ash is used to remove chemicals that cause. A combination of lime and soda ash, along with coagulant and flocculant chemicals, is added to raw water to promote a precipitation reaction. Softening often removes iron and manganese, reduces tastes and odors, reduces total solids content, and removes radioactivity. As in the chlorination process, lime reacts with substances in the. How Does Lime Softening Work.
From www.youtube.com
Hot LimeSoda Process LimeSoda Process water treatment plant How Does Lime Softening Work Softening often removes iron and manganese, reduces tastes and odors, reduces total solids content, and removes radioactivity. This allows softening to take place. A combination of lime and soda ash, along with coagulant and flocculant chemicals, is added to raw water to promote a precipitation reaction. As in the chlorination process, lime reacts with substances in the water before it. How Does Lime Softening Work.
From waterfiltersadvisor.com
Drinking Softened Water (HowTo Guide) Methods WaterFiltersAdvisor How Does Lime Softening Work A combination of lime and soda ash, along with coagulant and flocculant chemicals, is added to raw water to promote a precipitation reaction. The high ph associated with lime softening can aid in. Softening often removes iron and manganese, reduces tastes and odors, reduces total solids content, and removes radioactivity. As in the chlorination process, lime reacts with substances in. How Does Lime Softening Work.
From homewater101.com
How Does a Water Softener Work? Water Softening Process Diagram How Does Lime Softening Work This allows softening to take place. As in the chlorination process, lime reacts with substances in the water before it can begin softening the water. Soda ash is used to remove chemicals that cause. Softening often removes iron and manganese, reduces tastes and odors, reduces total solids content, and removes radioactivity. Lime is used to remove chemicals that cause carbonate. How Does Lime Softening Work.
From contendo.ca
Hot Lime Softener (HLS) Process Flows SAGD Oil Sands Online Training How Does Lime Softening Work Soda ash is used to remove chemicals that cause. Lime is used to remove chemicals that cause carbonate hardness. The high ph associated with lime softening can aid in. This allows softening to take place. As in the chlorination process, lime reacts with substances in the water before it can begin softening the water. Softening often removes iron and manganese,. How Does Lime Softening Work.
From www.slideserve.com
PPT Lime Softening PowerPoint Presentation, free download ID2529217 How Does Lime Softening Work Soda ash is used to remove chemicals that cause. Lime is used to remove chemicals that cause carbonate hardness. Learn about lime softening in this excerpt from the lime softening lecture from our water treatment exam prep course visit our website. A combination of lime and soda ash, along with coagulant and flocculant chemicals, is added to raw water to. How Does Lime Softening Work.
From www.youtube.com
Lime Soda method for softening of water YouTube How Does Lime Softening Work Lime is used to remove chemicals that cause carbonate hardness. A combination of lime and soda ash, along with coagulant and flocculant chemicals, is added to raw water to promote a precipitation reaction. The high ph associated with lime softening can aid in. This allows softening to take place. Soda ash is used to remove chemicals that cause. As in. How Does Lime Softening Work.
From www.slideserve.com
PPT Effective Lime/Soda Ash Water Softening PowerPoint Presentation How Does Lime Softening Work A combination of lime and soda ash, along with coagulant and flocculant chemicals, is added to raw water to promote a precipitation reaction. Soda ash is used to remove chemicals that cause. Softening often removes iron and manganese, reduces tastes and odors, reduces total solids content, and removes radioactivity. Lime is used to remove chemicals that cause carbonate hardness. The. How Does Lime Softening Work.
From www.youtube.com
Water softening Lime soda method Zeolite process for water How Does Lime Softening Work As in the chlorination process, lime reacts with substances in the water before it can begin softening the water. Learn about lime softening in this excerpt from the lime softening lecture from our water treatment exam prep course visit our website. This allows softening to take place. Lime is used to remove chemicals that cause carbonate hardness. A combination of. How Does Lime Softening Work.
From issuu.com
Lime Softening—the Technology Optimization Case Studies From How Does Lime Softening Work As in the chlorination process, lime reacts with substances in the water before it can begin softening the water. Lime is used to remove chemicals that cause carbonate hardness. Learn about lime softening in this excerpt from the lime softening lecture from our water treatment exam prep course visit our website. The high ph associated with lime softening can aid. How Does Lime Softening Work.
From www.youtube.com
ENE 483 Lime Softening Example Calculating Stoichiometric Amounts of How Does Lime Softening Work Lime is used to remove chemicals that cause carbonate hardness. The high ph associated with lime softening can aid in. A combination of lime and soda ash, along with coagulant and flocculant chemicals, is added to raw water to promote a precipitation reaction. This allows softening to take place. Soda ash is used to remove chemicals that cause. As in. How Does Lime Softening Work.
From www.youtube.com
ENE 483 Lime Soda Ash Softening Split Treatment YouTube How Does Lime Softening Work Lime is used to remove chemicals that cause carbonate hardness. This allows softening to take place. Learn about lime softening in this excerpt from the lime softening lecture from our water treatment exam prep course visit our website. A combination of lime and soda ash, along with coagulant and flocculant chemicals, is added to raw water to promote a precipitation. How Does Lime Softening Work.
From netsolwater.com
What is cold lime softening Netsol Water How Does Lime Softening Work As in the chlorination process, lime reacts with substances in the water before it can begin softening the water. Lime is used to remove chemicals that cause carbonate hardness. This allows softening to take place. The high ph associated with lime softening can aid in. Soda ash is used to remove chemicals that cause. Softening often removes iron and manganese,. How Does Lime Softening Work.
From www.youtube.com
Lime Soda ash Softening YouTube How Does Lime Softening Work A combination of lime and soda ash, along with coagulant and flocculant chemicals, is added to raw water to promote a precipitation reaction. As in the chlorination process, lime reacts with substances in the water before it can begin softening the water. Softening often removes iron and manganese, reduces tastes and odors, reduces total solids content, and removes radioactivity. The. How Does Lime Softening Work.
From www.slideserve.com
PPT Water Treatment Part 3 Groundwater Treatment PowerPoint How Does Lime Softening Work This allows softening to take place. Soda ash is used to remove chemicals that cause. As in the chlorination process, lime reacts with substances in the water before it can begin softening the water. Softening often removes iron and manganese, reduces tastes and odors, reduces total solids content, and removes radioactivity. A combination of lime and soda ash, along with. How Does Lime Softening Work.