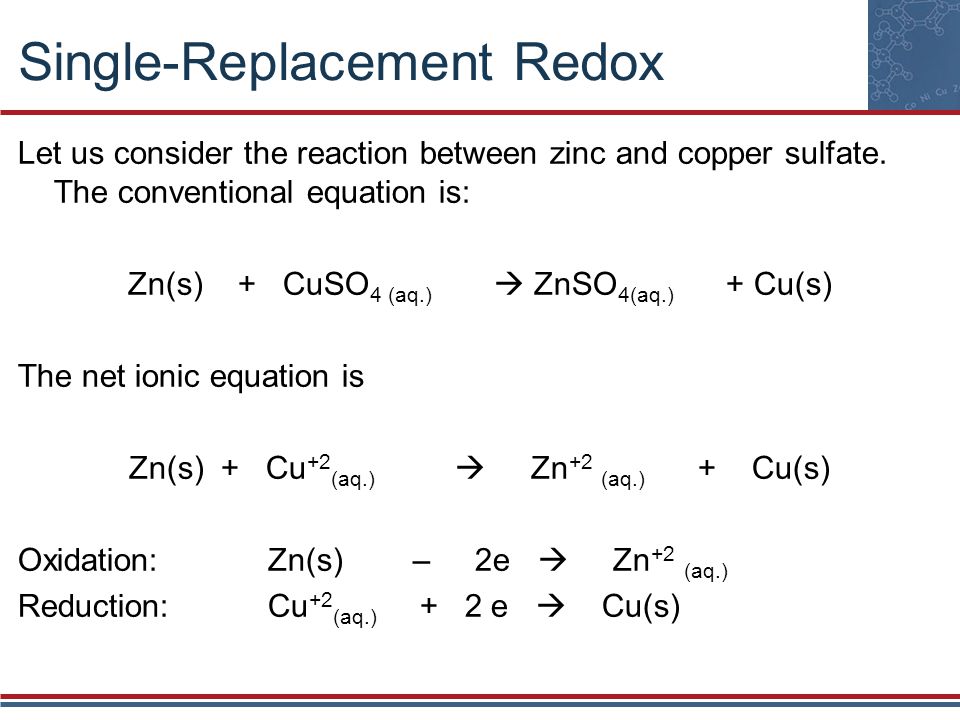
Zinc Electroplating Chemical Reactions . The results show that zn 2+ exists in the alkaline solution in the form of zn (oh) 2− 4. In this lab you will be electroplating zinc onto a penny, copper onto a nickel, and calculating the number of moles of each metal deposited onto. The apparent activation energy of the electrode reaction is. The experiments were designed to explore the galvanic zinc coating process under a constant voltage of 4 v, with varying immersion times set at intervals of 5 min, 25 min,. There are three different types of zinc plating processes: The electrodeposition of zinc has found use in many fields, from.
from schoolworkhelper.net
The apparent activation energy of the electrode reaction is. There are three different types of zinc plating processes: The electrodeposition of zinc has found use in many fields, from. The experiments were designed to explore the galvanic zinc coating process under a constant voltage of 4 v, with varying immersion times set at intervals of 5 min, 25 min,. In this lab you will be electroplating zinc onto a penny, copper onto a nickel, and calculating the number of moles of each metal deposited onto. The results show that zn 2+ exists in the alkaline solution in the form of zn (oh) 2− 4.
Single Displacement Reactions Lab Explained SchoolWorkHelper
Zinc Electroplating Chemical Reactions The experiments were designed to explore the galvanic zinc coating process under a constant voltage of 4 v, with varying immersion times set at intervals of 5 min, 25 min,. In this lab you will be electroplating zinc onto a penny, copper onto a nickel, and calculating the number of moles of each metal deposited onto. The electrodeposition of zinc has found use in many fields, from. The experiments were designed to explore the galvanic zinc coating process under a constant voltage of 4 v, with varying immersion times set at intervals of 5 min, 25 min,. The apparent activation energy of the electrode reaction is. The results show that zn 2+ exists in the alkaline solution in the form of zn (oh) 2− 4. There are three different types of zinc plating processes:
From www.youtube.com
R3.2.16 Electroplating (HL) YouTube Zinc Electroplating Chemical Reactions In this lab you will be electroplating zinc onto a penny, copper onto a nickel, and calculating the number of moles of each metal deposited onto. There are three different types of zinc plating processes: The electrodeposition of zinc has found use in many fields, from. The results show that zn 2+ exists in the alkaline solution in the form. Zinc Electroplating Chemical Reactions.
From www.wiringdraw.com
Draw A Simple Circuit Diagram To Show Electroplating Zinc Electroplating Chemical Reactions In this lab you will be electroplating zinc onto a penny, copper onto a nickel, and calculating the number of moles of each metal deposited onto. The experiments were designed to explore the galvanic zinc coating process under a constant voltage of 4 v, with varying immersion times set at intervals of 5 min, 25 min,. There are three different. Zinc Electroplating Chemical Reactions.
From ceg.edu.vn
Share more than 138 electroplating iron nail with copper ceg.edu.vn Zinc Electroplating Chemical Reactions The results show that zn 2+ exists in the alkaline solution in the form of zn (oh) 2− 4. The apparent activation energy of the electrode reaction is. The experiments were designed to explore the galvanic zinc coating process under a constant voltage of 4 v, with varying immersion times set at intervals of 5 min, 25 min,. The electrodeposition. Zinc Electroplating Chemical Reactions.
From brainly.in
Explain the process of electroplating with the help of a labelled Zinc Electroplating Chemical Reactions The results show that zn 2+ exists in the alkaline solution in the form of zn (oh) 2− 4. In this lab you will be electroplating zinc onto a penny, copper onto a nickel, and calculating the number of moles of each metal deposited onto. The experiments were designed to explore the galvanic zinc coating process under a constant voltage. Zinc Electroplating Chemical Reactions.
From www.youtube.com
Zinc Electroplating by a Chemist an effective anti rust solution Zinc Electroplating Chemical Reactions The results show that zn 2+ exists in the alkaline solution in the form of zn (oh) 2− 4. There are three different types of zinc plating processes: The apparent activation energy of the electrode reaction is. The experiments were designed to explore the galvanic zinc coating process under a constant voltage of 4 v, with varying immersion times set. Zinc Electroplating Chemical Reactions.
From mantavya.com
What Is Electroplating & How does it work 2021 Guide Mantavya Zinc Electroplating Chemical Reactions The electrodeposition of zinc has found use in many fields, from. The apparent activation energy of the electrode reaction is. There are three different types of zinc plating processes: The results show that zn 2+ exists in the alkaline solution in the form of zn (oh) 2− 4. In this lab you will be electroplating zinc onto a penny, copper. Zinc Electroplating Chemical Reactions.
From sites.google.com
Electrochemistry engineering chemistry Zinc Electroplating Chemical Reactions The apparent activation energy of the electrode reaction is. There are three different types of zinc plating processes: The electrodeposition of zinc has found use in many fields, from. The experiments were designed to explore the galvanic zinc coating process under a constant voltage of 4 v, with varying immersion times set at intervals of 5 min, 25 min,. In. Zinc Electroplating Chemical Reactions.
From www.youtube.com
Zn+H2O=Zn(OH)2+H2 Balanced EquationZinc+Water=Zinc hydroxide+Water Zinc Electroplating Chemical Reactions In this lab you will be electroplating zinc onto a penny, copper onto a nickel, and calculating the number of moles of each metal deposited onto. The apparent activation energy of the electrode reaction is. The electrodeposition of zinc has found use in many fields, from. The results show that zn 2+ exists in the alkaline solution in the form. Zinc Electroplating Chemical Reactions.
From www.youtube.com
XI Chemistry Ch07 Lec02 (Determination of electrode potential of zinc Zinc Electroplating Chemical Reactions The experiments were designed to explore the galvanic zinc coating process under a constant voltage of 4 v, with varying immersion times set at intervals of 5 min, 25 min,. The electrodeposition of zinc has found use in many fields, from. There are three different types of zinc plating processes: The results show that zn 2+ exists in the alkaline. Zinc Electroplating Chemical Reactions.
From www.degruyter.com
Sacrificial ZnNi coatings by electroplating and hydrogen embrittlement Zinc Electroplating Chemical Reactions There are three different types of zinc plating processes: In this lab you will be electroplating zinc onto a penny, copper onto a nickel, and calculating the number of moles of each metal deposited onto. The results show that zn 2+ exists in the alkaline solution in the form of zn (oh) 2− 4. The experiments were designed to explore. Zinc Electroplating Chemical Reactions.
From www.nagwa.com
Question Video Writing the Equation for the Reaction at the Anode Zinc Electroplating Chemical Reactions The electrodeposition of zinc has found use in many fields, from. The experiments were designed to explore the galvanic zinc coating process under a constant voltage of 4 v, with varying immersion times set at intervals of 5 min, 25 min,. There are three different types of zinc plating processes: The results show that zn 2+ exists in the alkaline. Zinc Electroplating Chemical Reactions.
From sensorex.com
Electroplating The Process & Uses in Liquid Analysis Explained Sensorex Zinc Electroplating Chemical Reactions The electrodeposition of zinc has found use in many fields, from. The results show that zn 2+ exists in the alkaline solution in the form of zn (oh) 2− 4. The apparent activation energy of the electrode reaction is. There are three different types of zinc plating processes: In this lab you will be electroplating zinc onto a penny, copper. Zinc Electroplating Chemical Reactions.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Zinc Electroplating Chemical Reactions The apparent activation energy of the electrode reaction is. There are three different types of zinc plating processes: In this lab you will be electroplating zinc onto a penny, copper onto a nickel, and calculating the number of moles of each metal deposited onto. The electrodeposition of zinc has found use in many fields, from. The results show that zn. Zinc Electroplating Chemical Reactions.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Zinc Electroplating Chemical Reactions There are three different types of zinc plating processes: The experiments were designed to explore the galvanic zinc coating process under a constant voltage of 4 v, with varying immersion times set at intervals of 5 min, 25 min,. The apparent activation energy of the electrode reaction is. The electrodeposition of zinc has found use in many fields, from. In. Zinc Electroplating Chemical Reactions.
From saylordotorg.github.io
Describing Electrochemical Cells Zinc Electroplating Chemical Reactions The experiments were designed to explore the galvanic zinc coating process under a constant voltage of 4 v, with varying immersion times set at intervals of 5 min, 25 min,. In this lab you will be electroplating zinc onto a penny, copper onto a nickel, and calculating the number of moles of each metal deposited onto. The results show that. Zinc Electroplating Chemical Reactions.
From www.youtube.com
Electroplating of Zinc 9th Chemistry Chap 7 Electrochemistry in Zinc Electroplating Chemical Reactions There are three different types of zinc plating processes: The experiments were designed to explore the galvanic zinc coating process under a constant voltage of 4 v, with varying immersion times set at intervals of 5 min, 25 min,. In this lab you will be electroplating zinc onto a penny, copper onto a nickel, and calculating the number of moles. Zinc Electroplating Chemical Reactions.
From www.wiringdraw.com
Draw A Simple Circuit Diagram To Show Electroplating And Answer The Zinc Electroplating Chemical Reactions The electrodeposition of zinc has found use in many fields, from. The apparent activation energy of the electrode reaction is. The experiments were designed to explore the galvanic zinc coating process under a constant voltage of 4 v, with varying immersion times set at intervals of 5 min, 25 min,. The results show that zn 2+ exists in the alkaline. Zinc Electroplating Chemical Reactions.
From www.chemistrylearner.com
Electroplating Definition, Process, Example, and Equation Zinc Electroplating Chemical Reactions The apparent activation energy of the electrode reaction is. In this lab you will be electroplating zinc onto a penny, copper onto a nickel, and calculating the number of moles of each metal deposited onto. The electrodeposition of zinc has found use in many fields, from. There are three different types of zinc plating processes: The results show that zn. Zinc Electroplating Chemical Reactions.
From www.animalia-life.club
Chromium Plating Zinc Electroplating Chemical Reactions In this lab you will be electroplating zinc onto a penny, copper onto a nickel, and calculating the number of moles of each metal deposited onto. The apparent activation energy of the electrode reaction is. The experiments were designed to explore the galvanic zinc coating process under a constant voltage of 4 v, with varying immersion times set at intervals. Zinc Electroplating Chemical Reactions.
From www.vlr.eng.br
Electroplating Apparatus vlr.eng.br Zinc Electroplating Chemical Reactions The results show that zn 2+ exists in the alkaline solution in the form of zn (oh) 2− 4. There are three different types of zinc plating processes: In this lab you will be electroplating zinc onto a penny, copper onto a nickel, and calculating the number of moles of each metal deposited onto. The apparent activation energy of the. Zinc Electroplating Chemical Reactions.
From bsbgroup.com
Corrosion protection of structural steel Brewer Smith Brewer Group Zinc Electroplating Chemical Reactions The apparent activation energy of the electrode reaction is. The electrodeposition of zinc has found use in many fields, from. The results show that zn 2+ exists in the alkaline solution in the form of zn (oh) 2− 4. There are three different types of zinc plating processes: In this lab you will be electroplating zinc onto a penny, copper. Zinc Electroplating Chemical Reactions.
From www.mdpi.com
Coatings Free FullText Effects of Organic Additives on Alkaline Zinc Electroplating Chemical Reactions There are three different types of zinc plating processes: The electrodeposition of zinc has found use in many fields, from. The results show that zn 2+ exists in the alkaline solution in the form of zn (oh) 2− 4. In this lab you will be electroplating zinc onto a penny, copper onto a nickel, and calculating the number of moles. Zinc Electroplating Chemical Reactions.
From letstalkscience.ca
Chemical Reactions Conditions & Speeds Let's Talk Science Zinc Electroplating Chemical Reactions The results show that zn 2+ exists in the alkaline solution in the form of zn (oh) 2− 4. The experiments were designed to explore the galvanic zinc coating process under a constant voltage of 4 v, with varying immersion times set at intervals of 5 min, 25 min,. In this lab you will be electroplating zinc onto a penny,. Zinc Electroplating Chemical Reactions.
From ar.inspiredpencil.com
Zinc Electroplating Diagram Zinc Electroplating Chemical Reactions There are three different types of zinc plating processes: The results show that zn 2+ exists in the alkaline solution in the form of zn (oh) 2− 4. The experiments were designed to explore the galvanic zinc coating process under a constant voltage of 4 v, with varying immersion times set at intervals of 5 min, 25 min,. In this. Zinc Electroplating Chemical Reactions.
From courses.lumenlearning.com
Electrolysis Chemistry Atoms First Zinc Electroplating Chemical Reactions The experiments were designed to explore the galvanic zinc coating process under a constant voltage of 4 v, with varying immersion times set at intervals of 5 min, 25 min,. There are three different types of zinc plating processes: The results show that zn 2+ exists in the alkaline solution in the form of zn (oh) 2− 4. In this. Zinc Electroplating Chemical Reactions.
From www.researchgate.net
Reactions performed during the nickel electroplating process Download Zinc Electroplating Chemical Reactions There are three different types of zinc plating processes: The electrodeposition of zinc has found use in many fields, from. The apparent activation energy of the electrode reaction is. The experiments were designed to explore the galvanic zinc coating process under a constant voltage of 4 v, with varying immersion times set at intervals of 5 min, 25 min,. The. Zinc Electroplating Chemical Reactions.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Zinc Electroplating Chemical Reactions In this lab you will be electroplating zinc onto a penny, copper onto a nickel, and calculating the number of moles of each metal deposited onto. The results show that zn 2+ exists in the alkaline solution in the form of zn (oh) 2− 4. The experiments were designed to explore the galvanic zinc coating process under a constant voltage. Zinc Electroplating Chemical Reactions.
From www.researchgate.net
Schematic illustration of the zinc phosphate mechanism with passive Zinc Electroplating Chemical Reactions The electrodeposition of zinc has found use in many fields, from. The experiments were designed to explore the galvanic zinc coating process under a constant voltage of 4 v, with varying immersion times set at intervals of 5 min, 25 min,. The results show that zn 2+ exists in the alkaline solution in the form of zn (oh) 2− 4.. Zinc Electroplating Chemical Reactions.
From www.aakash.ac.in
Redox Reactions Definition, Types, Applications & Uses Chemistry Zinc Electroplating Chemical Reactions In this lab you will be electroplating zinc onto a penny, copper onto a nickel, and calculating the number of moles of each metal deposited onto. There are three different types of zinc plating processes: The electrodeposition of zinc has found use in many fields, from. The experiments were designed to explore the galvanic zinc coating process under a constant. Zinc Electroplating Chemical Reactions.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Zinc Electroplating Chemical Reactions The results show that zn 2+ exists in the alkaline solution in the form of zn (oh) 2− 4. The experiments were designed to explore the galvanic zinc coating process under a constant voltage of 4 v, with varying immersion times set at intervals of 5 min, 25 min,. The apparent activation energy of the electrode reaction is. In this. Zinc Electroplating Chemical Reactions.
From www.climate-policy-watcher.org
Electrolytic Silver Recovery Industrial Wastes Zinc Electroplating Chemical Reactions The electrodeposition of zinc has found use in many fields, from. The apparent activation energy of the electrode reaction is. The results show that zn 2+ exists in the alkaline solution in the form of zn (oh) 2− 4. In this lab you will be electroplating zinc onto a penny, copper onto a nickel, and calculating the number of moles. Zinc Electroplating Chemical Reactions.
From www.sliderbase.com
Electrochemical Terminology Zinc Electroplating Chemical Reactions The results show that zn 2+ exists in the alkaline solution in the form of zn (oh) 2− 4. The experiments were designed to explore the galvanic zinc coating process under a constant voltage of 4 v, with varying immersion times set at intervals of 5 min, 25 min,. The apparent activation energy of the electrode reaction is. The electrodeposition. Zinc Electroplating Chemical Reactions.
From letstalkscience.ca
Chemical Reactions Conditions & Speeds Let's Talk Science Zinc Electroplating Chemical Reactions In this lab you will be electroplating zinc onto a penny, copper onto a nickel, and calculating the number of moles of each metal deposited onto. The apparent activation energy of the electrode reaction is. The results show that zn 2+ exists in the alkaline solution in the form of zn (oh) 2− 4. The experiments were designed to explore. Zinc Electroplating Chemical Reactions.
From blog.thepipingmart.com
Zinc and Copper Redox Reaction Equation Zinc Electroplating Chemical Reactions The apparent activation energy of the electrode reaction is. There are three different types of zinc plating processes: The results show that zn 2+ exists in the alkaline solution in the form of zn (oh) 2− 4. The experiments were designed to explore the galvanic zinc coating process under a constant voltage of 4 v, with varying immersion times set. Zinc Electroplating Chemical Reactions.
From www.builderscalculator.com
Galvanized vs Zinc Plated Steel Builder's Calculator Zinc Electroplating Chemical Reactions The apparent activation energy of the electrode reaction is. There are three different types of zinc plating processes: The electrodeposition of zinc has found use in many fields, from. The results show that zn 2+ exists in the alkaline solution in the form of zn (oh) 2− 4. In this lab you will be electroplating zinc onto a penny, copper. Zinc Electroplating Chemical Reactions.