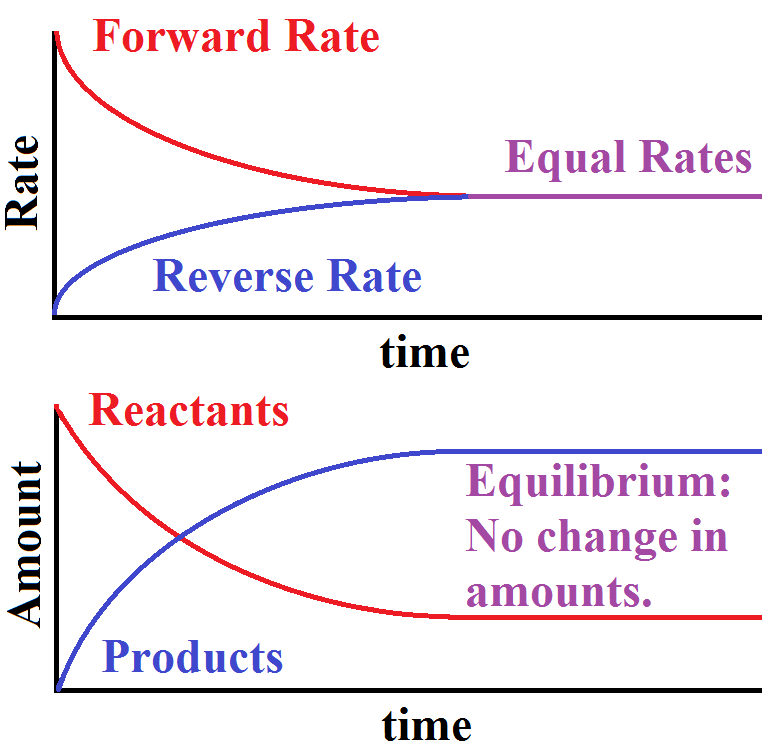
Equilibrium Constant Chemistry Definition . The equilibrium constant, , represents the extent of a reaction when it is at equilibrium. relate the magnitude of an equilibrium constant to properties of the chemical system. derive reaction quotients from chemical equations representing homogeneous and heterogeneous reactions. Now that we have a symbol (⇌). Express reaction quotients for chemical equations representing homogeneous and. what does it represent? the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. It uses the concentrations and. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. the equilibrium constant is the ratio of the equilibrium concentrations of the products raised to the power of their stoichiometric.
from giozqbmjw.blob.core.windows.net
The equilibrium constant, , represents the extent of a reaction when it is at equilibrium. the equilibrium constant is the ratio of the equilibrium concentrations of the products raised to the power of their stoichiometric. It uses the concentrations and. what does it represent? the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. relate the magnitude of an equilibrium constant to properties of the chemical system. derive reaction quotients from chemical equations representing homogeneous and heterogeneous reactions. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. Now that we have a symbol (⇌). Express reaction quotients for chemical equations representing homogeneous and.
Catalyst Chemical Equilibrium Constant at Vanessa Benites blog
Equilibrium Constant Chemistry Definition the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. what does it represent? Now that we have a symbol (⇌). relate the magnitude of an equilibrium constant to properties of the chemical system. derive reaction quotients from chemical equations representing homogeneous and heterogeneous reactions. The equilibrium constant, , represents the extent of a reaction when it is at equilibrium. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. the equilibrium constant is the ratio of the equilibrium concentrations of the products raised to the power of their stoichiometric. Express reaction quotients for chemical equations representing homogeneous and. It uses the concentrations and.
From secondaryscience4all.wordpress.com
1.10 Equilibrium constant Kc for homogeneous systems (Equilibrium A2 Equilibrium Constant Chemistry Definition Now that we have a symbol (⇌). relate the magnitude of an equilibrium constant to properties of the chemical system. what does it represent? the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. The equilibrium constant, , represents the extent of a reaction when it is at. Equilibrium Constant Chemistry Definition.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM Chapter 16 PowerPoint Presentation, free Equilibrium Constant Chemistry Definition Express reaction quotients for chemical equations representing homogeneous and. what does it represent? relate the magnitude of an equilibrium constant to properties of the chemical system. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. It uses the concentrations and. Now that we have. Equilibrium Constant Chemistry Definition.
From giozqbmjw.blob.core.windows.net
Catalyst Chemical Equilibrium Constant at Vanessa Benites blog Equilibrium Constant Chemistry Definition relate the magnitude of an equilibrium constant to properties of the chemical system. The equilibrium constant, , represents the extent of a reaction when it is at equilibrium. Now that we have a symbol (⇌). the equilibrium constant is the ratio of the equilibrium concentrations of the products raised to the power of their stoichiometric. the equilibrium. Equilibrium Constant Chemistry Definition.
From mungfali.com
Types Of Equilibrium Chemistry Equilibrium Constant Chemistry Definition the equilibrium constant is the ratio of the equilibrium concentrations of the products raised to the power of their stoichiometric. Express reaction quotients for chemical equations representing homogeneous and. It uses the concentrations and. Now that we have a symbol (⇌). derive reaction quotients from chemical equations representing homogeneous and heterogeneous reactions. relate the magnitude of an. Equilibrium Constant Chemistry Definition.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Equilibrium Constant Chemistry Definition the equilibrium constant is the ratio of the equilibrium concentrations of the products raised to the power of their stoichiometric. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. Now that we have a symbol (⇌). the equilibrium constant is equal to the rate constant for the. Equilibrium Constant Chemistry Definition.
From www.vrogue.co
Dynamic Chemical Equilibrium Definition Examples Vide vrogue.co Equilibrium Constant Chemistry Definition the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. Now that we have a symbol (⇌). It uses the concentrations and. The equilibrium constant, , represents the extent of a reaction when it is at equilibrium. derive reaction quotients from chemical equations representing homogeneous and heterogeneous reactions. . Equilibrium Constant Chemistry Definition.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Equilibrium Constant Chemistry Definition Express reaction quotients for chemical equations representing homogeneous and. derive reaction quotients from chemical equations representing homogeneous and heterogeneous reactions. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. the equilibrium constant is the ratio of the equilibrium concentrations of the products raised to the power of. Equilibrium Constant Chemistry Definition.
From thechemistrynotes.com
Equilibrium Constant Definition, Characteristics, and Calculations Equilibrium Constant Chemistry Definition The equilibrium constant, , represents the extent of a reaction when it is at equilibrium. what does it represent? derive reaction quotients from chemical equations representing homogeneous and heterogeneous reactions. Now that we have a symbol (⇌). the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the. Equilibrium Constant Chemistry Definition.
From mungfali.com
Types Of Equilibrium Chemistry Equilibrium Constant Chemistry Definition derive reaction quotients from chemical equations representing homogeneous and heterogeneous reactions. Express reaction quotients for chemical equations representing homogeneous and. what does it represent? the equilibrium constant is the ratio of the equilibrium concentrations of the products raised to the power of their stoichiometric. the equilibrium constant, k, expresses the relationship between products and reactants of. Equilibrium Constant Chemistry Definition.
From www.slideserve.com
PPT Chapter 15 Chemical Equilibrium PowerPoint Presentation, free Equilibrium Constant Chemistry Definition Express reaction quotients for chemical equations representing homogeneous and. The equilibrium constant, , represents the extent of a reaction when it is at equilibrium. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. Now that we have a symbol (⇌). relate the magnitude of an equilibrium constant to. Equilibrium Constant Chemistry Definition.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Equilibrium Constant Chemistry Definition what does it represent? the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. relate the magnitude of an equilibrium constant to properties of the chemical system. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with. Equilibrium Constant Chemistry Definition.
From www.youtube.com
Chemical Equilibrium and the Equilibrium Constant YouTube Equilibrium Constant Chemistry Definition derive reaction quotients from chemical equations representing homogeneous and heterogeneous reactions. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. what does it represent? The equilibrium constant, , represents the extent of a reaction when it is at equilibrium. It uses the concentrations and. relate the. Equilibrium Constant Chemistry Definition.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Equilibrium Constant Chemistry Definition what does it represent? relate the magnitude of an equilibrium constant to properties of the chemical system. It uses the concentrations and. Now that we have a symbol (⇌). the equilibrium constant is the ratio of the equilibrium concentrations of the products raised to the power of their stoichiometric. Express reaction quotients for chemical equations representing homogeneous. Equilibrium Constant Chemistry Definition.
From www.slideserve.com
PPT Equilibrium Chemistry PowerPoint Presentation, free download ID Equilibrium Constant Chemistry Definition The equilibrium constant, , represents the extent of a reaction when it is at equilibrium. Express reaction quotients for chemical equations representing homogeneous and. Now that we have a symbol (⇌). It uses the concentrations and. what does it represent? the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant. Equilibrium Constant Chemistry Definition.
From www.slideserve.com
PPT Introduction to Chemical Equilibrium PowerPoint Presentation Equilibrium Constant Chemistry Definition derive reaction quotients from chemical equations representing homogeneous and heterogeneous reactions. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. relate the magnitude of an equilibrium constant to properties of the chemical system. It uses the concentrations and. what does it represent? Express. Equilibrium Constant Chemistry Definition.
From www.slideserve.com
PPT Chemical Equilibrium CHAPTER 15 Chemistry The Molecular Nature Equilibrium Constant Chemistry Definition Now that we have a symbol (⇌). Express reaction quotients for chemical equations representing homogeneous and. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. relate the magnitude of an equilibrium constant to properties of the chemical system. the equilibrium constant is the ratio of the equilibrium. Equilibrium Constant Chemistry Definition.
From www.youtube.com
The equilibrium expression and the equilibrium constant (Keq) YouTube Equilibrium Constant Chemistry Definition relate the magnitude of an equilibrium constant to properties of the chemical system. Express reaction quotients for chemical equations representing homogeneous and. Now that we have a symbol (⇌). the equilibrium constant is the ratio of the equilibrium concentrations of the products raised to the power of their stoichiometric. It uses the concentrations and. derive reaction quotients. Equilibrium Constant Chemistry Definition.
From learningchemistryeasily.blogspot.com
Learning Chemistry Easily Chemical Equilibrium Theory, An Introduction Equilibrium Constant Chemistry Definition what does it represent? relate the magnitude of an equilibrium constant to properties of the chemical system. Now that we have a symbol (⇌). The equilibrium constant, , represents the extent of a reaction when it is at equilibrium. It uses the concentrations and. the equilibrium constant is equal to the rate constant for the forward reaction. Equilibrium Constant Chemistry Definition.
From www.youtube.com
Understanding The Equilibrium Constant, Kp (A2 Chemistry) YouTube Equilibrium Constant Chemistry Definition Express reaction quotients for chemical equations representing homogeneous and. It uses the concentrations and. relate the magnitude of an equilibrium constant to properties of the chemical system. Now that we have a symbol (⇌). the equilibrium constant is the ratio of the equilibrium concentrations of the products raised to the power of their stoichiometric. the equilibrium constant. Equilibrium Constant Chemistry Definition.
From www.youtube.com
10th Chemistry Chapter 9 Chemical equilibrium Equilibrium Constant Equilibrium Constant Chemistry Definition It uses the concentrations and. what does it represent? the equilibrium constant is the ratio of the equilibrium concentrations of the products raised to the power of their stoichiometric. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. The equilibrium constant, , represents the extent of a. Equilibrium Constant Chemistry Definition.
From www.showme.com
The equilibrium constant is a constant! Science, Chemistry, Physical Equilibrium Constant Chemistry Definition what does it represent? Now that we have a symbol (⇌). the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. relate the magnitude of an equilibrium constant to properties of the chemical system. Express reaction quotients for chemical equations representing homogeneous and. the. Equilibrium Constant Chemistry Definition.
From pediaa.com
Difference Between Reaction Quotient and Equilibrium Constant Equilibrium Constant Chemistry Definition relate the magnitude of an equilibrium constant to properties of the chemical system. Express reaction quotients for chemical equations representing homogeneous and. derive reaction quotients from chemical equations representing homogeneous and heterogeneous reactions. the equilibrium constant is the ratio of the equilibrium concentrations of the products raised to the power of their stoichiometric. the equilibrium constant. Equilibrium Constant Chemistry Definition.
From gamesmartz.com
Equilibrium Constant Definition & Image GameSmartz Equilibrium Constant Chemistry Definition derive reaction quotients from chemical equations representing homogeneous and heterogeneous reactions. Express reaction quotients for chemical equations representing homogeneous and. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. It uses the concentrations and. Now that we have a symbol (⇌). relate the magnitude. Equilibrium Constant Chemistry Definition.
From general.chemistrysteps.com
Equilibrium Constant Chemistry Steps Equilibrium Constant Chemistry Definition Express reaction quotients for chemical equations representing homogeneous and. derive reaction quotients from chemical equations representing homogeneous and heterogeneous reactions. relate the magnitude of an equilibrium constant to properties of the chemical system. the equilibrium constant is the ratio of the equilibrium concentrations of the products raised to the power of their stoichiometric. the equilibrium constant. Equilibrium Constant Chemistry Definition.
From www.pinterest.com
K = The Equilibrium Constant. [A] = Molarity of A in the solution. Ap Equilibrium Constant Chemistry Definition the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. It uses the concentrations and. derive reaction quotients from chemical equations representing homogeneous and heterogeneous reactions. the equilibrium constant is the ratio of the equilibrium concentrations of the products raised to the power of their stoichiometric. the. Equilibrium Constant Chemistry Definition.
From www.youtube.com
EQUILIBRIUM CONSTANT AND UNIT PART 1 / CONTEXT OF CHEMISTRY YouTube Equilibrium Constant Chemistry Definition derive reaction quotients from chemical equations representing homogeneous and heterogeneous reactions. It uses the concentrations and. Express reaction quotients for chemical equations representing homogeneous and. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. the equilibrium constant is the ratio of the equilibrium concentrations. Equilibrium Constant Chemistry Definition.
From www.len.com.ng
Chemical Equilibrium Dynamic Equilibrium in Chemistry Equilibrium Constant Chemistry Definition derive reaction quotients from chemical equations representing homogeneous and heterogeneous reactions. Express reaction quotients for chemical equations representing homogeneous and. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. what does it represent? The equilibrium constant, , represents the extent of a reaction when it is at. Equilibrium Constant Chemistry Definition.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Equilibrium Constant Chemistry Definition derive reaction quotients from chemical equations representing homogeneous and heterogeneous reactions. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. relate the magnitude of. Equilibrium Constant Chemistry Definition.
From teagan-yersblogcombs.blogspot.com
An Equilibrium Constant Is Described by Which of the Following Equilibrium Constant Chemistry Definition the equilibrium constant is the ratio of the equilibrium concentrations of the products raised to the power of their stoichiometric. derive reaction quotients from chemical equations representing homogeneous and heterogeneous reactions. It uses the concentrations and. Express reaction quotients for chemical equations representing homogeneous and. The equilibrium constant, , represents the extent of a reaction when it is. Equilibrium Constant Chemistry Definition.
From classnotes.org.in
Characteristics Of Equilibrium Constant Chemistry, Class 11, Equilibrium Equilibrium Constant Chemistry Definition Express reaction quotients for chemical equations representing homogeneous and. what does it represent? Now that we have a symbol (⇌). the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant. Equilibrium Constant Chemistry Definition.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Equilibrium Constant Chemistry Definition relate the magnitude of an equilibrium constant to properties of the chemical system. It uses the concentrations and. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction. Express reaction quotients for chemical equations representing homogeneous and. derive reaction quotients from chemical equations representing homogeneous. Equilibrium Constant Chemistry Definition.
From www.slideserve.com
PPT EQUILIBRIUM CONSTANTS Kp PowerPoint Presentation, free download Equilibrium Constant Chemistry Definition the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. the equilibrium constant is the ratio of the equilibrium concentrations of the products raised to the power of their stoichiometric. Now that we have a symbol (⇌). derive reaction quotients from chemical equations representing homogeneous and heterogeneous reactions.. Equilibrium Constant Chemistry Definition.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Equilibrium Constant Chemistry Definition the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. the equilibrium constant is the ratio of the equilibrium concentrations of the products raised to the power of their stoichiometric. relate the magnitude of an equilibrium constant to properties of the chemical system. derive reaction quotients from. Equilibrium Constant Chemistry Definition.
From www.slideserve.com
PPT The Equilibrium Condition, the Equilibrium Constant and Equilibrium Constant Chemistry Definition the equilibrium constant is the ratio of the equilibrium concentrations of the products raised to the power of their stoichiometric. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. the equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant. Equilibrium Constant Chemistry Definition.
From www.pinterest.com
Chemical Equilibrium Types ,Principles and Laws of Equilibria Equilibrium Constant Chemistry Definition what does it represent? the equilibrium constant is the ratio of the equilibrium concentrations of the products raised to the power of their stoichiometric. relate the magnitude of an equilibrium constant to properties of the chemical system. The equilibrium constant, , represents the extent of a reaction when it is at equilibrium. the equilibrium constant, k,. Equilibrium Constant Chemistry Definition.