Example Of Condensation Reaction In The Human Body . It is a synthesis reaction and substitution reaction. biomolecular condensates are cellular structures composed of membraneless assemblies comprising. in chemistry, a condensation reaction is an organic chemical reaction in which two or more reactants combine to form a single product, accompanied by the loss of a small molecule such as water, an alcohol, or an acid. condensation is the reaction that forms large, biological molecules. a condensation reaction occurs when two molecules join to form a larger molecule and release a smaller molecule (s) in the process. The smaller molecule lost in the reaction is often water, but it can also be methanol, hydrogen chloride, acetic acid or several other molecules. an example of a biomolecular condensate is a stress granule, which assembles in stressed cells from rna and. dehydration synthesis is also called as “condensation reaction” because both are characterized by the condensation and formation of water from the large molecule. revision notes on 1.4 condensation & hydrolysis for the edexcel international a level biology syllabus, written by the. Examples of dehydration synthesis reactions are the conversion of monosaccharides to complex sugars, production of proteins from amino acids, conversion of fatty acids. The reaction releases water and bonds the smaller components together into larger.
from www.slideserve.com
condensation is the reaction that forms large, biological molecules. It is a synthesis reaction and substitution reaction. an example of a biomolecular condensate is a stress granule, which assembles in stressed cells from rna and. biomolecular condensates are cellular structures composed of membraneless assemblies comprising. dehydration synthesis is also called as “condensation reaction” because both are characterized by the condensation and formation of water from the large molecule. Examples of dehydration synthesis reactions are the conversion of monosaccharides to complex sugars, production of proteins from amino acids, conversion of fatty acids. a condensation reaction occurs when two molecules join to form a larger molecule and release a smaller molecule (s) in the process. The smaller molecule lost in the reaction is often water, but it can also be methanol, hydrogen chloride, acetic acid or several other molecules. The reaction releases water and bonds the smaller components together into larger. revision notes on 1.4 condensation & hydrolysis for the edexcel international a level biology syllabus, written by the.
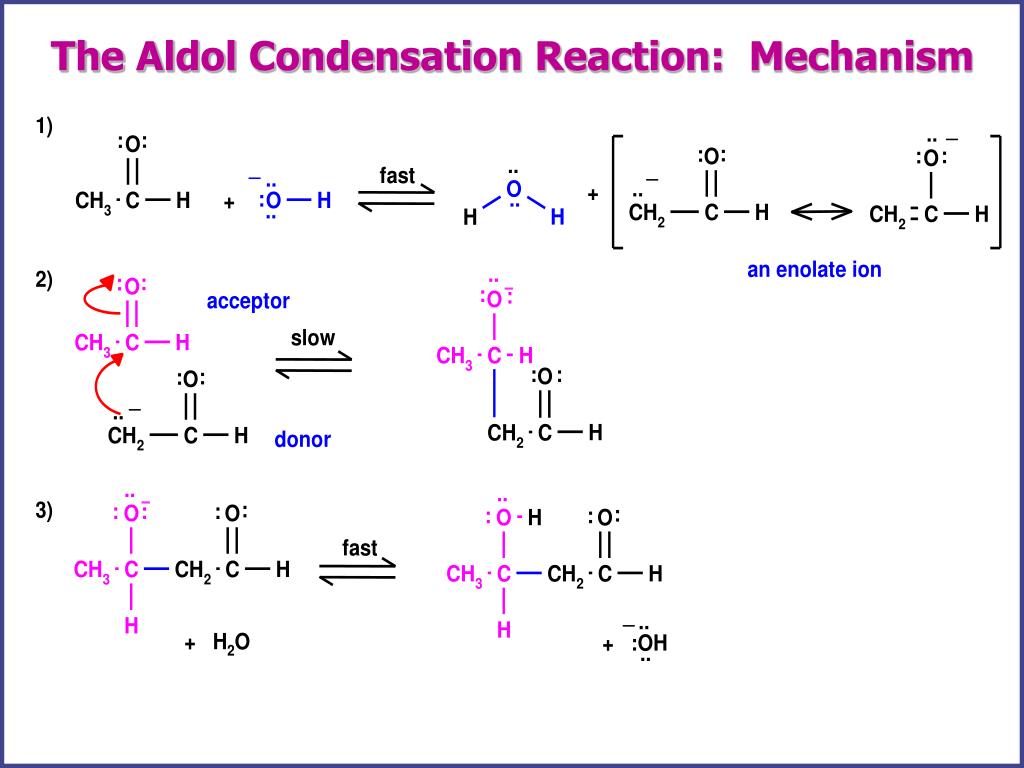
PPT The Aldol Condensation PowerPoint Presentation, free download
Example Of Condensation Reaction In The Human Body The smaller molecule lost in the reaction is often water, but it can also be methanol, hydrogen chloride, acetic acid or several other molecules. biomolecular condensates are cellular structures composed of membraneless assemblies comprising. an example of a biomolecular condensate is a stress granule, which assembles in stressed cells from rna and. Examples of dehydration synthesis reactions are the conversion of monosaccharides to complex sugars, production of proteins from amino acids, conversion of fatty acids. dehydration synthesis is also called as “condensation reaction” because both are characterized by the condensation and formation of water from the large molecule. It is a synthesis reaction and substitution reaction. revision notes on 1.4 condensation & hydrolysis for the edexcel international a level biology syllabus, written by the. condensation is the reaction that forms large, biological molecules. The reaction releases water and bonds the smaller components together into larger. a condensation reaction occurs when two molecules join to form a larger molecule and release a smaller molecule (s) in the process. in chemistry, a condensation reaction is an organic chemical reaction in which two or more reactants combine to form a single product, accompanied by the loss of a small molecule such as water, an alcohol, or an acid. The smaller molecule lost in the reaction is often water, but it can also be methanol, hydrogen chloride, acetic acid or several other molecules.
From theeducationlife.com
Another Name For A Condensation Reaction The Education Example Of Condensation Reaction In The Human Body dehydration synthesis is also called as “condensation reaction” because both are characterized by the condensation and formation of water from the large molecule. an example of a biomolecular condensate is a stress granule, which assembles in stressed cells from rna and. a condensation reaction occurs when two molecules join to form a larger molecule and release a. Example Of Condensation Reaction In The Human Body.
From chemistnotes.com
Dieckmann Condensation Mechanism, Examples and Application Chemistry Example Of Condensation Reaction In The Human Body Examples of dehydration synthesis reactions are the conversion of monosaccharides to complex sugars, production of proteins from amino acids, conversion of fatty acids. an example of a biomolecular condensate is a stress granule, which assembles in stressed cells from rna and. condensation is the reaction that forms large, biological molecules. The reaction releases water and bonds the smaller. Example Of Condensation Reaction In The Human Body.
From www.slideserve.com
PPT Biochemistry Part 2 PowerPoint Presentation, free download ID Example Of Condensation Reaction In The Human Body dehydration synthesis is also called as “condensation reaction” because both are characterized by the condensation and formation of water from the large molecule. condensation is the reaction that forms large, biological molecules. an example of a biomolecular condensate is a stress granule, which assembles in stressed cells from rna and. The smaller molecule lost in the reaction. Example Of Condensation Reaction In The Human Body.
From www.slideserve.com
PPT Key Concepts PowerPoint Presentation, free download ID358483 Example Of Condensation Reaction In The Human Body dehydration synthesis is also called as “condensation reaction” because both are characterized by the condensation and formation of water from the large molecule. The smaller molecule lost in the reaction is often water, but it can also be methanol, hydrogen chloride, acetic acid or several other molecules. condensation is the reaction that forms large, biological molecules. in. Example Of Condensation Reaction In The Human Body.
From studylib.net
Condensation and Hydrolysis Example Of Condensation Reaction In The Human Body condensation is the reaction that forms large, biological molecules. dehydration synthesis is also called as “condensation reaction” because both are characterized by the condensation and formation of water from the large molecule. It is a synthesis reaction and substitution reaction. The reaction releases water and bonds the smaller components together into larger. biomolecular condensates are cellular structures. Example Of Condensation Reaction In The Human Body.
From www.coursehero.com
[Solved] 1. Describe a condensation reaction. Illustrate one example Example Of Condensation Reaction In The Human Body The smaller molecule lost in the reaction is often water, but it can also be methanol, hydrogen chloride, acetic acid or several other molecules. Examples of dehydration synthesis reactions are the conversion of monosaccharides to complex sugars, production of proteins from amino acids, conversion of fatty acids. a condensation reaction occurs when two molecules join to form a larger. Example Of Condensation Reaction In The Human Body.
From www.slideserve.com
PPT Changes in State of Matter PowerPoint Presentation, free download Example Of Condensation Reaction In The Human Body biomolecular condensates are cellular structures composed of membraneless assemblies comprising. a condensation reaction occurs when two molecules join to form a larger molecule and release a smaller molecule (s) in the process. in chemistry, a condensation reaction is an organic chemical reaction in which two or more reactants combine to form a single product, accompanied by the. Example Of Condensation Reaction In The Human Body.
From sciencing.com
What is a Condensation Reaction? Sciencing Example Of Condensation Reaction In The Human Body The reaction releases water and bonds the smaller components together into larger. It is a synthesis reaction and substitution reaction. dehydration synthesis is also called as “condensation reaction” because both are characterized by the condensation and formation of water from the large molecule. biomolecular condensates are cellular structures composed of membraneless assemblies comprising. in chemistry, a condensation. Example Of Condensation Reaction In The Human Body.
From missehonorsbio.blogspot.com
EC Honors Biology Condensation reactions Example Of Condensation Reaction In The Human Body The reaction releases water and bonds the smaller components together into larger. a condensation reaction occurs when two molecules join to form a larger molecule and release a smaller molecule (s) in the process. dehydration synthesis is also called as “condensation reaction” because both are characterized by the condensation and formation of water from the large molecule. . Example Of Condensation Reaction In The Human Body.
From www.thoughtco.com
Condensation Reaction Definition in Chemistry Example Of Condensation Reaction In The Human Body It is a synthesis reaction and substitution reaction. Examples of dehydration synthesis reactions are the conversion of monosaccharides to complex sugars, production of proteins from amino acids, conversion of fatty acids. revision notes on 1.4 condensation & hydrolysis for the edexcel international a level biology syllabus, written by the. biomolecular condensates are cellular structures composed of membraneless assemblies. Example Of Condensation Reaction In The Human Body.
From www.youtube.com
Condensation Process Real life examples of Condensation Daily life Example Of Condensation Reaction In The Human Body revision notes on 1.4 condensation & hydrolysis for the edexcel international a level biology syllabus, written by the. biomolecular condensates are cellular structures composed of membraneless assemblies comprising. It is a synthesis reaction and substitution reaction. The smaller molecule lost in the reaction is often water, but it can also be methanol, hydrogen chloride, acetic acid or several. Example Of Condensation Reaction In The Human Body.
From www.slideshare.net
Condensation hydrolysis Example Of Condensation Reaction In The Human Body biomolecular condensates are cellular structures composed of membraneless assemblies comprising. in chemistry, a condensation reaction is an organic chemical reaction in which two or more reactants combine to form a single product, accompanied by the loss of a small molecule such as water, an alcohol, or an acid. revision notes on 1.4 condensation & hydrolysis for the. Example Of Condensation Reaction In The Human Body.
From www.slideserve.com
PPT Biochemistry PowerPoint Presentation, free download ID9134641 Example Of Condensation Reaction In The Human Body condensation is the reaction that forms large, biological molecules. in chemistry, a condensation reaction is an organic chemical reaction in which two or more reactants combine to form a single product, accompanied by the loss of a small molecule such as water, an alcohol, or an acid. It is a synthesis reaction and substitution reaction. a condensation. Example Of Condensation Reaction In The Human Body.
From slideplayer.com
Organic Reactions. ppt download Example Of Condensation Reaction In The Human Body biomolecular condensates are cellular structures composed of membraneless assemblies comprising. The smaller molecule lost in the reaction is often water, but it can also be methanol, hydrogen chloride, acetic acid or several other molecules. an example of a biomolecular condensate is a stress granule, which assembles in stressed cells from rna and. condensation is the reaction that. Example Of Condensation Reaction In The Human Body.
From chemistnotes.com
Perkin Condensation Mechanism and Applications Chemistry Notes Example Of Condensation Reaction In The Human Body The smaller molecule lost in the reaction is often water, but it can also be methanol, hydrogen chloride, acetic acid or several other molecules. a condensation reaction occurs when two molecules join to form a larger molecule and release a smaller molecule (s) in the process. dehydration synthesis is also called as “condensation reaction” because both are characterized. Example Of Condensation Reaction In The Human Body.
From www.dynamicscience.com.au
biologycondensation reactions. Example Of Condensation Reaction In The Human Body biomolecular condensates are cellular structures composed of membraneless assemblies comprising. condensation is the reaction that forms large, biological molecules. The smaller molecule lost in the reaction is often water, but it can also be methanol, hydrogen chloride, acetic acid or several other molecules. revision notes on 1.4 condensation & hydrolysis for the edexcel international a level biology. Example Of Condensation Reaction In The Human Body.
From animalia-life.club
Draw A Condensation Reaction Example Of Condensation Reaction In The Human Body revision notes on 1.4 condensation & hydrolysis for the edexcel international a level biology syllabus, written by the. It is a synthesis reaction and substitution reaction. an example of a biomolecular condensate is a stress granule, which assembles in stressed cells from rna and. The reaction releases water and bonds the smaller components together into larger. The smaller. Example Of Condensation Reaction In The Human Body.
From open.oregonstate.education
2.4 Compounds Essential to Human Functioning Anatomy Example Of Condensation Reaction In The Human Body biomolecular condensates are cellular structures composed of membraneless assemblies comprising. in chemistry, a condensation reaction is an organic chemical reaction in which two or more reactants combine to form a single product, accompanied by the loss of a small molecule such as water, an alcohol, or an acid. a condensation reaction occurs when two molecules join to. Example Of Condensation Reaction In The Human Body.
From www.slideserve.com
PPT The Aldol Condensation PowerPoint Presentation, free download Example Of Condensation Reaction In The Human Body revision notes on 1.4 condensation & hydrolysis for the edexcel international a level biology syllabus, written by the. dehydration synthesis is also called as “condensation reaction” because both are characterized by the condensation and formation of water from the large molecule. biomolecular condensates are cellular structures composed of membraneless assemblies comprising. condensation is the reaction that. Example Of Condensation Reaction In The Human Body.
From sciencenotes.org
Condensation Reaction Definition and Examples Example Of Condensation Reaction In The Human Body The reaction releases water and bonds the smaller components together into larger. condensation is the reaction that forms large, biological molecules. dehydration synthesis is also called as “condensation reaction” because both are characterized by the condensation and formation of water from the large molecule. The smaller molecule lost in the reaction is often water, but it can also. Example Of Condensation Reaction In The Human Body.
From www.youtube.com
Claisen Condensation Reaction Definition, Mechanism with Examples Example Of Condensation Reaction In The Human Body The reaction releases water and bonds the smaller components together into larger. in chemistry, a condensation reaction is an organic chemical reaction in which two or more reactants combine to form a single product, accompanied by the loss of a small molecule such as water, an alcohol, or an acid. revision notes on 1.4 condensation & hydrolysis for. Example Of Condensation Reaction In The Human Body.
From missehonorsbio.blogspot.com
EC Honors Biology Condensation reactions Example Of Condensation Reaction In The Human Body in chemistry, a condensation reaction is an organic chemical reaction in which two or more reactants combine to form a single product, accompanied by the loss of a small molecule such as water, an alcohol, or an acid. It is a synthesis reaction and substitution reaction. dehydration synthesis is also called as “condensation reaction” because both are characterized. Example Of Condensation Reaction In The Human Body.
From www.coursehero.com
[Solved] 1. Describe a condensation reaction. Illustrate one example Example Of Condensation Reaction In The Human Body biomolecular condensates are cellular structures composed of membraneless assemblies comprising. condensation is the reaction that forms large, biological molecules. an example of a biomolecular condensate is a stress granule, which assembles in stressed cells from rna and. revision notes on 1.4 condensation & hydrolysis for the edexcel international a level biology syllabus, written by the. . Example Of Condensation Reaction In The Human Body.
From www.youtube.com
Dieckmann Condensation Reaction Complete Mechanism and Examples Example Of Condensation Reaction In The Human Body Examples of dehydration synthesis reactions are the conversion of monosaccharides to complex sugars, production of proteins from amino acids, conversion of fatty acids. dehydration synthesis is also called as “condensation reaction” because both are characterized by the condensation and formation of water from the large molecule. It is a synthesis reaction and substitution reaction. an example of a. Example Of Condensation Reaction In The Human Body.
From www.researchgate.net
Different condensation reactions relevant for the formation of Example Of Condensation Reaction In The Human Body in chemistry, a condensation reaction is an organic chemical reaction in which two or more reactants combine to form a single product, accompanied by the loss of a small molecule such as water, an alcohol, or an acid. The smaller molecule lost in the reaction is often water, but it can also be methanol, hydrogen chloride, acetic acid or. Example Of Condensation Reaction In The Human Body.
From chemistry-reaction.com
Perkin Reaction (Perkin Condensation) definition mechanism example Example Of Condensation Reaction In The Human Body condensation is the reaction that forms large, biological molecules. It is a synthesis reaction and substitution reaction. biomolecular condensates are cellular structures composed of membraneless assemblies comprising. revision notes on 1.4 condensation & hydrolysis for the edexcel international a level biology syllabus, written by the. The smaller molecule lost in the reaction is often water, but it. Example Of Condensation Reaction In The Human Body.
From www.slideserve.com
PPT Basic Organc Chemistry III PowerPoint Presentation, free download Example Of Condensation Reaction In The Human Body in chemistry, a condensation reaction is an organic chemical reaction in which two or more reactants combine to form a single product, accompanied by the loss of a small molecule such as water, an alcohol, or an acid. revision notes on 1.4 condensation & hydrolysis for the edexcel international a level biology syllabus, written by the. The reaction. Example Of Condensation Reaction In The Human Body.
From mavink.com
Condensation Reaction A Level Biology Example Of Condensation Reaction In The Human Body revision notes on 1.4 condensation & hydrolysis for the edexcel international a level biology syllabus, written by the. Examples of dehydration synthesis reactions are the conversion of monosaccharides to complex sugars, production of proteins from amino acids, conversion of fatty acids. It is a synthesis reaction and substitution reaction. a condensation reaction occurs when two molecules join to. Example Of Condensation Reaction In The Human Body.
From www.researchgate.net
VSS+ example The process of condensation Download Scientific Diagram Example Of Condensation Reaction In The Human Body a condensation reaction occurs when two molecules join to form a larger molecule and release a smaller molecule (s) in the process. It is a synthesis reaction and substitution reaction. revision notes on 1.4 condensation & hydrolysis for the edexcel international a level biology syllabus, written by the. dehydration synthesis is also called as “condensation reaction” because. Example Of Condensation Reaction In The Human Body.
From www.slideserve.com
PPT Molecules of Life PowerPoint Presentation, free download ID6955153 Example Of Condensation Reaction In The Human Body condensation is the reaction that forms large, biological molecules. The smaller molecule lost in the reaction is often water, but it can also be methanol, hydrogen chloride, acetic acid or several other molecules. dehydration synthesis is also called as “condensation reaction” because both are characterized by the condensation and formation of water from the large molecule. revision. Example Of Condensation Reaction In The Human Body.
From www.slideserve.com
PPT Biochemistry PowerPoint Presentation, free download ID2308033 Example Of Condensation Reaction In The Human Body a condensation reaction occurs when two molecules join to form a larger molecule and release a smaller molecule (s) in the process. dehydration synthesis is also called as “condensation reaction” because both are characterized by the condensation and formation of water from the large molecule. revision notes on 1.4 condensation & hydrolysis for the edexcel international a. Example Of Condensation Reaction In The Human Body.
From chemistry-reaction.com
Acy loin Condensation Mechanism with examples Example Of Condensation Reaction In The Human Body The reaction releases water and bonds the smaller components together into larger. It is a synthesis reaction and substitution reaction. dehydration synthesis is also called as “condensation reaction” because both are characterized by the condensation and formation of water from the large molecule. revision notes on 1.4 condensation & hydrolysis for the edexcel international a level biology syllabus,. Example Of Condensation Reaction In The Human Body.
From slideplayer.com
The Chemical Basis of Life ppt download Example Of Condensation Reaction In The Human Body dehydration synthesis is also called as “condensation reaction” because both are characterized by the condensation and formation of water from the large molecule. The smaller molecule lost in the reaction is often water, but it can also be methanol, hydrogen chloride, acetic acid or several other molecules. in chemistry, a condensation reaction is an organic chemical reaction in. Example Of Condensation Reaction In The Human Body.
From study.com
Aldol Condensation Definition, Mechanism & Reaction Video & Lesson Example Of Condensation Reaction In The Human Body dehydration synthesis is also called as “condensation reaction” because both are characterized by the condensation and formation of water from the large molecule. condensation is the reaction that forms large, biological molecules. a condensation reaction occurs when two molecules join to form a larger molecule and release a smaller molecule (s) in the process. The smaller molecule. Example Of Condensation Reaction In The Human Body.
From www.slideserve.com
PPT The Aldol Condensation PowerPoint Presentation, free download Example Of Condensation Reaction In The Human Body The smaller molecule lost in the reaction is often water, but it can also be methanol, hydrogen chloride, acetic acid or several other molecules. Examples of dehydration synthesis reactions are the conversion of monosaccharides to complex sugars, production of proteins from amino acids, conversion of fatty acids. It is a synthesis reaction and substitution reaction. an example of a. Example Of Condensation Reaction In The Human Body.