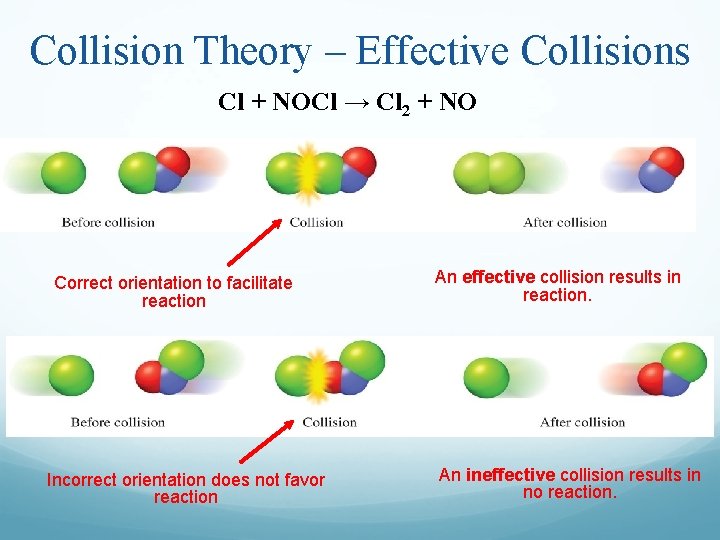
What Does A Collision Required To Be Effective . For a gas at room temperature. Molecules must collide with favourable spatial orientation. For a collision to be. They must have sufficient energy to overcome activation energy and the. effective collisions must meet two main criteria: effective collisions are the interactions between reactant molecules that result in a chemical reaction. collision theory explains how materials can collide and become new materials. Successful collision (also referred to as effective, or reactive collision) Effective collisions result in product formation. for collisions to be successful, reacting particles must (1) collide with (2) sufficient energy, and (3) with the proper orientation. according to the collision theory, two criteria must be met in order for a chemical reaction to take place: a collision will be effective in producing chemical change only if the species brought together possess a. an effective collision (b) is one in which chemical bonds are broken and a product is formed.
from slidetodoc.com
effective collisions are the interactions between reactant molecules that result in a chemical reaction. Molecules must collide with favourable spatial orientation. for collisions to be successful, reacting particles must (1) collide with (2) sufficient energy, and (3) with the proper orientation. Effective collisions result in product formation. Successful collision (also referred to as effective, or reactive collision) For a collision to be. They must have sufficient energy to overcome activation energy and the. For a gas at room temperature. according to the collision theory, two criteria must be met in order for a chemical reaction to take place: effective collisions must meet two main criteria:
Chapter 16 Chemical Equilibrium CHM 130 GCC Chemistry
What Does A Collision Required To Be Effective a collision will be effective in producing chemical change only if the species brought together possess a. according to the collision theory, two criteria must be met in order for a chemical reaction to take place: Successful collision (also referred to as effective, or reactive collision) a collision will be effective in producing chemical change only if the species brought together possess a. collision theory explains how materials can collide and become new materials. effective collisions are the interactions between reactant molecules that result in a chemical reaction. For a gas at room temperature. Effective collisions result in product formation. For a collision to be. Molecules must collide with favourable spatial orientation. They must have sufficient energy to overcome activation energy and the. for collisions to be successful, reacting particles must (1) collide with (2) sufficient energy, and (3) with the proper orientation. effective collisions must meet two main criteria: an effective collision (b) is one in which chemical bonds are broken and a product is formed.
From www.dlawgroup.com
What are the 3 Types of Collisions That Occur in a Car Crash? The 3 What Does A Collision Required To Be Effective according to the collision theory, two criteria must be met in order for a chemical reaction to take place: collision theory explains how materials can collide and become new materials. Successful collision (also referred to as effective, or reactive collision) effective collisions must meet two main criteria: effective collisions are the interactions between reactant molecules that. What Does A Collision Required To Be Effective.
From slideplayer.com
by Steven S. Zumdahl & Don J. DeCoste University of Illinois ppt download What Does A Collision Required To Be Effective collision theory explains how materials can collide and become new materials. for collisions to be successful, reacting particles must (1) collide with (2) sufficient energy, and (3) with the proper orientation. effective collisions are the interactions between reactant molecules that result in a chemical reaction. a collision will be effective in producing chemical change only if. What Does A Collision Required To Be Effective.
From aeftaha.blogspot.com
CHEMISTRY IS UNIVERSAL . CLASS WITH ME SEM 2 REACTION (PART 2) What Does A Collision Required To Be Effective They must have sufficient energy to overcome activation energy and the. For a gas at room temperature. for collisions to be successful, reacting particles must (1) collide with (2) sufficient energy, and (3) with the proper orientation. For a collision to be. Successful collision (also referred to as effective, or reactive collision) effective collisions must meet two main. What Does A Collision Required To Be Effective.
From www.slideserve.com
PPT Rates of Reaction PowerPoint Presentation, free download ID9519648 What Does A Collision Required To Be Effective collision theory explains how materials can collide and become new materials. Successful collision (also referred to as effective, or reactive collision) for collisions to be successful, reacting particles must (1) collide with (2) sufficient energy, and (3) with the proper orientation. Molecules must collide with favourable spatial orientation. effective collisions are the interactions between reactant molecules that. What Does A Collision Required To Be Effective.
From www.slideserve.com
PPT Unit V & Equilibrium PowerPoint Presentation ID5366818 What Does A Collision Required To Be Effective Effective collisions result in product formation. effective collisions are the interactions between reactant molecules that result in a chemical reaction. an effective collision (b) is one in which chemical bonds are broken and a product is formed. For a gas at room temperature. according to the collision theory, two criteria must be met in order for a. What Does A Collision Required To Be Effective.
From www.youtube.com
Collision Theory and How to Ensure Effective Collisions in Chemical What Does A Collision Required To Be Effective Effective collisions result in product formation. For a gas at room temperature. Molecules must collide with favourable spatial orientation. effective collisions must meet two main criteria: a collision will be effective in producing chemical change only if the species brought together possess a. according to the collision theory, two criteria must be met in order for a. What Does A Collision Required To Be Effective.
From slideplayer.com
Reaction Rates and Collision Theory ppt download What Does A Collision Required To Be Effective effective collisions must meet two main criteria: Effective collisions result in product formation. Successful collision (also referred to as effective, or reactive collision) collision theory explains how materials can collide and become new materials. a collision will be effective in producing chemical change only if the species brought together possess a. For a gas at room temperature.. What Does A Collision Required To Be Effective.
From www.youtube.com
Effective Collision Rate of Reaction YouTube What Does A Collision Required To Be Effective an effective collision (b) is one in which chemical bonds are broken and a product is formed. for collisions to be successful, reacting particles must (1) collide with (2) sufficient energy, and (3) with the proper orientation. effective collisions must meet two main criteria: For a collision to be. according to the collision theory, two criteria. What Does A Collision Required To Be Effective.
From slideplayer.com
THE COLLISION THEORY. ppt download What Does A Collision Required To Be Effective For a gas at room temperature. according to the collision theory, two criteria must be met in order for a chemical reaction to take place: For a collision to be. for collisions to be successful, reacting particles must (1) collide with (2) sufficient energy, and (3) with the proper orientation. Effective collisions result in product formation. effective. What Does A Collision Required To Be Effective.
From slideplayer.com
Explaining Chemical Changes ppt download What Does A Collision Required To Be Effective For a gas at room temperature. a collision will be effective in producing chemical change only if the species brought together possess a. They must have sufficient energy to overcome activation energy and the. effective collisions must meet two main criteria: effective collisions are the interactions between reactant molecules that result in a chemical reaction. for. What Does A Collision Required To Be Effective.
From enginelibgrizzles.z22.web.core.windows.net
How Does The Collision Theory Work What Does A Collision Required To Be Effective for collisions to be successful, reacting particles must (1) collide with (2) sufficient energy, and (3) with the proper orientation. an effective collision (b) is one in which chemical bonds are broken and a product is formed. Successful collision (also referred to as effective, or reactive collision) Molecules must collide with favourable spatial orientation. collision theory explains. What Does A Collision Required To Be Effective.
From www.slideserve.com
PPT Collision Theory PowerPoint Presentation, free download ID3873529 What Does A Collision Required To Be Effective For a gas at room temperature. a collision will be effective in producing chemical change only if the species brought together possess a. according to the collision theory, two criteria must be met in order for a chemical reaction to take place: for collisions to be successful, reacting particles must (1) collide with (2) sufficient energy, and. What Does A Collision Required To Be Effective.
From www.youtube.com
Part 4 Effective Collision YouTube What Does A Collision Required To Be Effective collision theory explains how materials can collide and become new materials. For a collision to be. according to the collision theory, two criteria must be met in order for a chemical reaction to take place: For a gas at room temperature. effective collisions are the interactions between reactant molecules that result in a chemical reaction. Molecules must. What Does A Collision Required To Be Effective.
From www.slideserve.com
PPT Collision Theory & Factors Affecting Reaction Rate PowerPoint What Does A Collision Required To Be Effective effective collisions are the interactions between reactant molecules that result in a chemical reaction. effective collisions must meet two main criteria: For a gas at room temperature. a collision will be effective in producing chemical change only if the species brought together possess a. Successful collision (also referred to as effective, or reactive collision) Effective collisions result. What Does A Collision Required To Be Effective.
From www.youtube.com
Speed of reactions effective collisions YouTube What Does A Collision Required To Be Effective They must have sufficient energy to overcome activation energy and the. For a collision to be. For a gas at room temperature. Effective collisions result in product formation. according to the collision theory, two criteria must be met in order for a chemical reaction to take place: an effective collision (b) is one in which chemical bonds are. What Does A Collision Required To Be Effective.
From slideplayer.com
and Chemical Equilibrium ppt download What Does A Collision Required To Be Effective a collision will be effective in producing chemical change only if the species brought together possess a. They must have sufficient energy to overcome activation energy and the. according to the collision theory, two criteria must be met in order for a chemical reaction to take place: an effective collision (b) is one in which chemical bonds. What Does A Collision Required To Be Effective.
From www.slideserve.com
PPT Chapter 9 PowerPoint Presentation, free download ID5697293 What Does A Collision Required To Be Effective effective collisions are the interactions between reactant molecules that result in a chemical reaction. Successful collision (also referred to as effective, or reactive collision) Molecules must collide with favourable spatial orientation. collision theory explains how materials can collide and become new materials. effective collisions must meet two main criteria: according to the collision theory, two criteria. What Does A Collision Required To Be Effective.
From slideplayer.com
Reaction Rates Reaction Rates Collision Theory ppt download What Does A Collision Required To Be Effective effective collisions must meet two main criteria: for collisions to be successful, reacting particles must (1) collide with (2) sufficient energy, and (3) with the proper orientation. collision theory explains how materials can collide and become new materials. Successful collision (also referred to as effective, or reactive collision) effective collisions are the interactions between reactant molecules. What Does A Collision Required To Be Effective.
From www.slideserve.com
PPT Reaction Rates and Equilibrium PowerPoint Presentation, free What Does A Collision Required To Be Effective collision theory explains how materials can collide and become new materials. Successful collision (also referred to as effective, or reactive collision) Molecules must collide with favourable spatial orientation. for collisions to be successful, reacting particles must (1) collide with (2) sufficient energy, and (3) with the proper orientation. effective collisions are the interactions between reactant molecules that. What Does A Collision Required To Be Effective.
From slideplayer.com
Lesson 3 Collision Theory. ppt download What Does A Collision Required To Be Effective for collisions to be successful, reacting particles must (1) collide with (2) sufficient energy, and (3) with the proper orientation. according to the collision theory, two criteria must be met in order for a chemical reaction to take place: They must have sufficient energy to overcome activation energy and the. Successful collision (also referred to as effective, or. What Does A Collision Required To Be Effective.
From mmerevise.co.uk
Collision Theory and Reaction Rates MME What Does A Collision Required To Be Effective an effective collision (b) is one in which chemical bonds are broken and a product is formed. according to the collision theory, two criteria must be met in order for a chemical reaction to take place: For a collision to be. Molecules must collide with favourable spatial orientation. for collisions to be successful, reacting particles must (1). What Does A Collision Required To Be Effective.
From slidetodoc.com
Collision Theory Collision Theory What is necessary for What Does A Collision Required To Be Effective For a collision to be. for collisions to be successful, reacting particles must (1) collide with (2) sufficient energy, and (3) with the proper orientation. Effective collisions result in product formation. Molecules must collide with favourable spatial orientation. Successful collision (also referred to as effective, or reactive collision) a collision will be effective in producing chemical change only. What Does A Collision Required To Be Effective.
From www.slideserve.com
PPT 1.3 Collision Theory PowerPoint Presentation, free download ID What Does A Collision Required To Be Effective effective collisions are the interactions between reactant molecules that result in a chemical reaction. collision theory explains how materials can collide and become new materials. effective collisions must meet two main criteria: for collisions to be successful, reacting particles must (1) collide with (2) sufficient energy, and (3) with the proper orientation. according to the. What Does A Collision Required To Be Effective.
From slideplayer.com
Reaction Rates and Collision Theory ppt download What Does A Collision Required To Be Effective according to the collision theory, two criteria must be met in order for a chemical reaction to take place: an effective collision (b) is one in which chemical bonds are broken and a product is formed. a collision will be effective in producing chemical change only if the species brought together possess a. For a gas at. What Does A Collision Required To Be Effective.
From www.slideserve.com
PPT Chapter 9 PowerPoint Presentation, free download ID5697293 What Does A Collision Required To Be Effective according to the collision theory, two criteria must be met in order for a chemical reaction to take place: collision theory explains how materials can collide and become new materials. effective collisions are the interactions between reactant molecules that result in a chemical reaction. For a gas at room temperature. Molecules must collide with favourable spatial orientation.. What Does A Collision Required To Be Effective.
From slideplayer.com
How do reactions occur? Must have an effective collision between What Does A Collision Required To Be Effective Effective collisions result in product formation. a collision will be effective in producing chemical change only if the species brought together possess a. effective collisions must meet two main criteria: For a collision to be. an effective collision (b) is one in which chemical bonds are broken and a product is formed. for collisions to be. What Does A Collision Required To Be Effective.
From slidetodoc.com
Chapter 16 Chemical Equilibrium CHM 130 GCC Chemistry What Does A Collision Required To Be Effective For a collision to be. They must have sufficient energy to overcome activation energy and the. according to the collision theory, two criteria must be met in order for a chemical reaction to take place: For a gas at room temperature. Effective collisions result in product formation. an effective collision (b) is one in which chemical bonds are. What Does A Collision Required To Be Effective.
From slideplayer.com
How do reactions occur? Must have an effective collision between What Does A Collision Required To Be Effective Effective collisions result in product formation. Successful collision (also referred to as effective, or reactive collision) collision theory explains how materials can collide and become new materials. effective collisions are the interactions between reactant molecules that result in a chemical reaction. effective collisions must meet two main criteria: For a gas at room temperature. For a collision. What Does A Collision Required To Be Effective.
From www.slideserve.com
PPT CHAPTER 9 Chemical Equilibrium PowerPoint Presentation, free What Does A Collision Required To Be Effective For a collision to be. effective collisions are the interactions between reactant molecules that result in a chemical reaction. Effective collisions result in product formation. Molecules must collide with favourable spatial orientation. an effective collision (b) is one in which chemical bonds are broken and a product is formed. For a gas at room temperature. collision theory. What Does A Collision Required To Be Effective.
From slideplayer.com
How do reactions occur? Must have an effective collision between What Does A Collision Required To Be Effective according to the collision theory, two criteria must be met in order for a chemical reaction to take place: for collisions to be successful, reacting particles must (1) collide with (2) sufficient energy, and (3) with the proper orientation. effective collisions must meet two main criteria: For a gas at room temperature. They must have sufficient energy. What Does A Collision Required To Be Effective.
From www.slideserve.com
PPT Thermochemistry Review PowerPoint Presentation, free download What Does A Collision Required To Be Effective a collision will be effective in producing chemical change only if the species brought together possess a. an effective collision (b) is one in which chemical bonds are broken and a product is formed. They must have sufficient energy to overcome activation energy and the. For a collision to be. collision theory explains how materials can collide. What Does A Collision Required To Be Effective.
From slideplayer.com
Factors that Affect Rate of Reaction ppt download What Does A Collision Required To Be Effective an effective collision (b) is one in which chemical bonds are broken and a product is formed. collision theory explains how materials can collide and become new materials. for collisions to be successful, reacting particles must (1) collide with (2) sufficient energy, and (3) with the proper orientation. They must have sufficient energy to overcome activation energy. What Does A Collision Required To Be Effective.
From studylib.net
Collisions What Does A Collision Required To Be Effective collision theory explains how materials can collide and become new materials. effective collisions are the interactions between reactant molecules that result in a chemical reaction. for collisions to be successful, reacting particles must (1) collide with (2) sufficient energy, and (3) with the proper orientation. according to the collision theory, two criteria must be met in. What Does A Collision Required To Be Effective.
From www.allstate.com
What is Collision Insurance? Allstate What Does A Collision Required To Be Effective For a gas at room temperature. Effective collisions result in product formation. Molecules must collide with favourable spatial orientation. effective collisions must meet two main criteria: For a collision to be. They must have sufficient energy to overcome activation energy and the. effective collisions are the interactions between reactant molecules that result in a chemical reaction. a. What Does A Collision Required To Be Effective.
From www.slideserve.com
PPT L8 (I) Physics of Collisions (II) Work and Energy PowerPoint What Does A Collision Required To Be Effective For a gas at room temperature. effective collisions must meet two main criteria: Successful collision (also referred to as effective, or reactive collision) collision theory explains how materials can collide and become new materials. Molecules must collide with favourable spatial orientation. For a collision to be. a collision will be effective in producing chemical change only if. What Does A Collision Required To Be Effective.