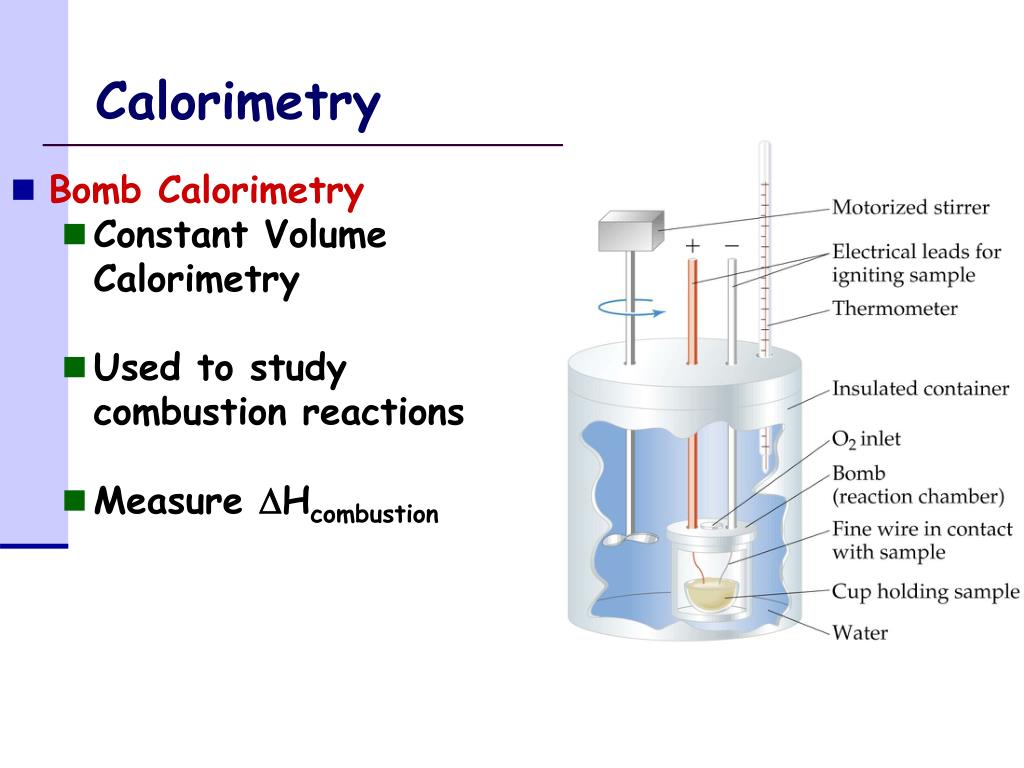
How Is Calorimetry Used In Real Life . Calorimetry is a crucial technique in thermodynamics, the study of heat transfer and energy conversion. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. For example, it's used in food science to determine the calorie content of. By knowing the change in. A calorimeter can be as simple. In this article, we will explore the definition of calorimeters in chemistry, their importance, types, and applications. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Essential to calorimetry is the calorimeter, which can be any device for accurately measuring the temperature of a substance before and after a change occurs. It is useful for several. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.
from www.slideserve.com
To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. In this article, we will explore the definition of calorimeters in chemistry, their importance, types, and applications. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. By knowing the change in. It is useful for several. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a crucial technique in thermodynamics, the study of heat transfer and energy conversion. Essential to calorimetry is the calorimeter, which can be any device for accurately measuring the temperature of a substance before and after a change occurs. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction.
PPT Calorimetry PowerPoint Presentation, free download ID6133898
How Is Calorimetry Used In Real Life In this article, we will explore the definition of calorimeters in chemistry, their importance, types, and applications. A calorimeter can be as simple. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. By knowing the change in. For example, it's used in food science to determine the calorie content of. Essential to calorimetry is the calorimeter, which can be any device for accurately measuring the temperature of a substance before and after a change occurs. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. It is useful for several. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. In this article, we will explore the definition of calorimeters in chemistry, their importance, types, and applications. Calorimetry is a crucial technique in thermodynamics, the study of heat transfer and energy conversion.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry How Is Calorimetry Used In Real Life Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. By knowing the change in. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter can be as. How Is Calorimetry Used In Real Life.
From www.slideshare.net
Lesson Enthalpy and Calorimetry How Is Calorimetry Used In Real Life Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Essential to calorimetry is the calorimeter, which can be any device for. How Is Calorimetry Used In Real Life.
From studylib.net
Calorimetry How Is Calorimetry Used In Real Life Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. In this article, we will explore the definition of calorimeters in chemistry, their importance, types, and applications.. How Is Calorimetry Used In Real Life.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL How Is Calorimetry Used In Real Life To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Calorimeters is. How Is Calorimetry Used In Real Life.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors How Is Calorimetry Used In Real Life To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. It is useful for several. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. A calorimeter can be as simple. In this article, we will explore the definition. How Is Calorimetry Used In Real Life.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 How Is Calorimetry Used In Real Life For example, it's used in food science to determine the calorie content of. A calorimeter can be as simple. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. How Is Calorimetry Used In Real Life.
From courses.lumenlearning.com
Calorimetry Introductory Chemistry Lecture & Lab How Is Calorimetry Used In Real Life It is useful for several. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. For example, it's used in food science to determine the. How Is Calorimetry Used In Real Life.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6655927 How Is Calorimetry Used In Real Life In this article, we will explore the definition of calorimeters in chemistry, their importance, types, and applications. Essential to calorimetry is the calorimeter, which can be any device for accurately measuring the temperature of a substance before and after a change occurs. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. It. How Is Calorimetry Used In Real Life.
From www.youtube.com
Types of calorimetry ?? Their advantages, Disadvantages & Use. YouTube How Is Calorimetry Used In Real Life It is useful for several. A calorimeter can be as simple. Essential to calorimetry is the calorimeter, which can be any device for accurately measuring the temperature of a substance before and after a change occurs. For example, it's used in food science to determine the calorie content of. Calorimeters is an important chemistry lab instrument devices that measure the. How Is Calorimetry Used In Real Life.
From study.com
Calorimetry Definition, Equation & Types Lesson How Is Calorimetry Used In Real Life Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter can be as simple. For example, it's used in food science to determine the calorie content of. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Essential to calorimetry. How Is Calorimetry Used In Real Life.
From giozuzdes.blob.core.windows.net
Examples Of Calorimetry In Real Life at Barbara Bader blog How Is Calorimetry Used In Real Life Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. A calorimeter can be as simple. For example, it's used in food science to determine the calorie content of. Calorimetry is. How Is Calorimetry Used In Real Life.
From saylordotorg.github.io
Calorimetry How Is Calorimetry Used In Real Life For example, it's used in food science to determine the calorie content of. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Essential to calorimetry is the calorimeter, which can be any device. How Is Calorimetry Used In Real Life.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 How Is Calorimetry Used In Real Life By knowing the change in. A calorimeter can be as simple. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimetry is a method of measuring. How Is Calorimetry Used In Real Life.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 How Is Calorimetry Used In Real Life Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is a crucial technique in thermodynamics, the study of heat transfer and energy conversion. Calorimetry is a method of measuring the heat transfer within a chemical reaction. How Is Calorimetry Used In Real Life.
From www.youtube.com
Calorimetry Part II Lab Version) YouTube How Is Calorimetry Used In Real Life Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Essential to calorimetry is the calorimeter, which can be any device for accurately measuring the temperature of a substance before and after a change occurs. For example, it's used in food science to determine the calorie content of.. How Is Calorimetry Used In Real Life.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle How Is Calorimetry Used In Real Life Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. For example, it's used in food science to determine the calorie content of. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical. How Is Calorimetry Used In Real Life.
From www.youtube.com
Calorimetry 2 Latent heat and real world applications by Venkatesh How Is Calorimetry Used In Real Life Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. In this article, we will explore the definition of calorimeters in chemistry, their importance, types, and applications. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating. How Is Calorimetry Used In Real Life.
From fyownpivb.blob.core.windows.net
What Is Calorimetry Used For In The Real World at Aline Nelson blog How Is Calorimetry Used In Real Life Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter can be as simple. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. It is useful for several. Essential to calorimetry is the calorimeter, which can be any device for accurately measuring the temperature of. How Is Calorimetry Used In Real Life.
From slideplayer.com
Calorimetry Chapter ppt download How Is Calorimetry Used In Real Life In this article, we will explore the definition of calorimeters in chemistry, their importance, types, and applications. Calorimetry is a crucial technique in thermodynamics, the study of heat transfer and energy conversion. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimeter, device for measuring. How Is Calorimetry Used In Real Life.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry How Is Calorimetry Used In Real Life For example, it's used in food science to determine the calorie content of. A calorimeter can be as simple. Calorimetry is a crucial technique in thermodynamics, the study of heat transfer and energy conversion. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter.. How Is Calorimetry Used In Real Life.
From courses.lumenlearning.com
Calorimetry Chemistry How Is Calorimetry Used In Real Life Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. By knowing the change in. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. How Is Calorimetry Used In Real Life.
From surfguppy.com
Enthalpy Surfguppy Chemistry made easy visual learning How Is Calorimetry Used In Real Life Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. For example,. How Is Calorimetry Used In Real Life.
From www.wisegeek.com
What is Calorimetry? (with pictures) How Is Calorimetry Used In Real Life Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter can be as simple. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. By knowing the change in. It is useful for several. Calorimeter,. How Is Calorimetry Used In Real Life.
From www.youtube.com
050 Calorimetry YouTube How Is Calorimetry Used In Real Life Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.. How Is Calorimetry Used In Real Life.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts How Is Calorimetry Used In Real Life Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. A calorimeter can be as simple. Calorimeters is an important chemistry lab instrument devices that measure the amount of. How Is Calorimetry Used In Real Life.
From www.thoughtco.com
Calorimeter Definition in Chemistry How Is Calorimetry Used In Real Life Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a. How Is Calorimetry Used In Real Life.
From faqguide.co
What does a calorimeter do? Explained by FAQGuide How Is Calorimetry Used In Real Life Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. For example, it's used in food science to determine the calorie content of. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Calorimetry is a branch. How Is Calorimetry Used In Real Life.
From studylib.net
Calorimetry How Is Calorimetry Used In Real Life Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. It is useful for several. Calorimetry is a crucial technique in thermodynamics,. How Is Calorimetry Used In Real Life.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube How Is Calorimetry Used In Real Life Calorimetry is a crucial technique in thermodynamics, the study of heat transfer and energy conversion. Essential to calorimetry is the calorimeter, which can be any device for accurately measuring the temperature of a substance before and after a change occurs. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. How Is Calorimetry Used In Real Life.
From slideplayer.com
Calorimetry CP Unit 9 Chapter ppt download How Is Calorimetry Used In Real Life Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. It is useful for several. For example, it's used in food science to determine the calorie content of. Calorimetry is a crucial technique in thermodynamics, the study of heat transfer and energy conversion. In this article, we will explore. How Is Calorimetry Used In Real Life.
From saylordotorg.github.io
Calorimetry How Is Calorimetry Used In Real Life By knowing the change in. A calorimeter can be as simple. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. For example, it's used in food science to determine the calorie content of. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used.. How Is Calorimetry Used In Real Life.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors How Is Calorimetry Used In Real Life Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes.. How Is Calorimetry Used In Real Life.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica How Is Calorimetry Used In Real Life Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a. How Is Calorimetry Used In Real Life.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson How Is Calorimetry Used In Real Life Calorimetry is a crucial technique in thermodynamics, the study of heat transfer and energy conversion. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Essential to. How Is Calorimetry Used In Real Life.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 How Is Calorimetry Used In Real Life To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. In this article, we will explore the definition of calorimeters in chemistry, their importance, types, and applications. By knowing. How Is Calorimetry Used In Real Life.