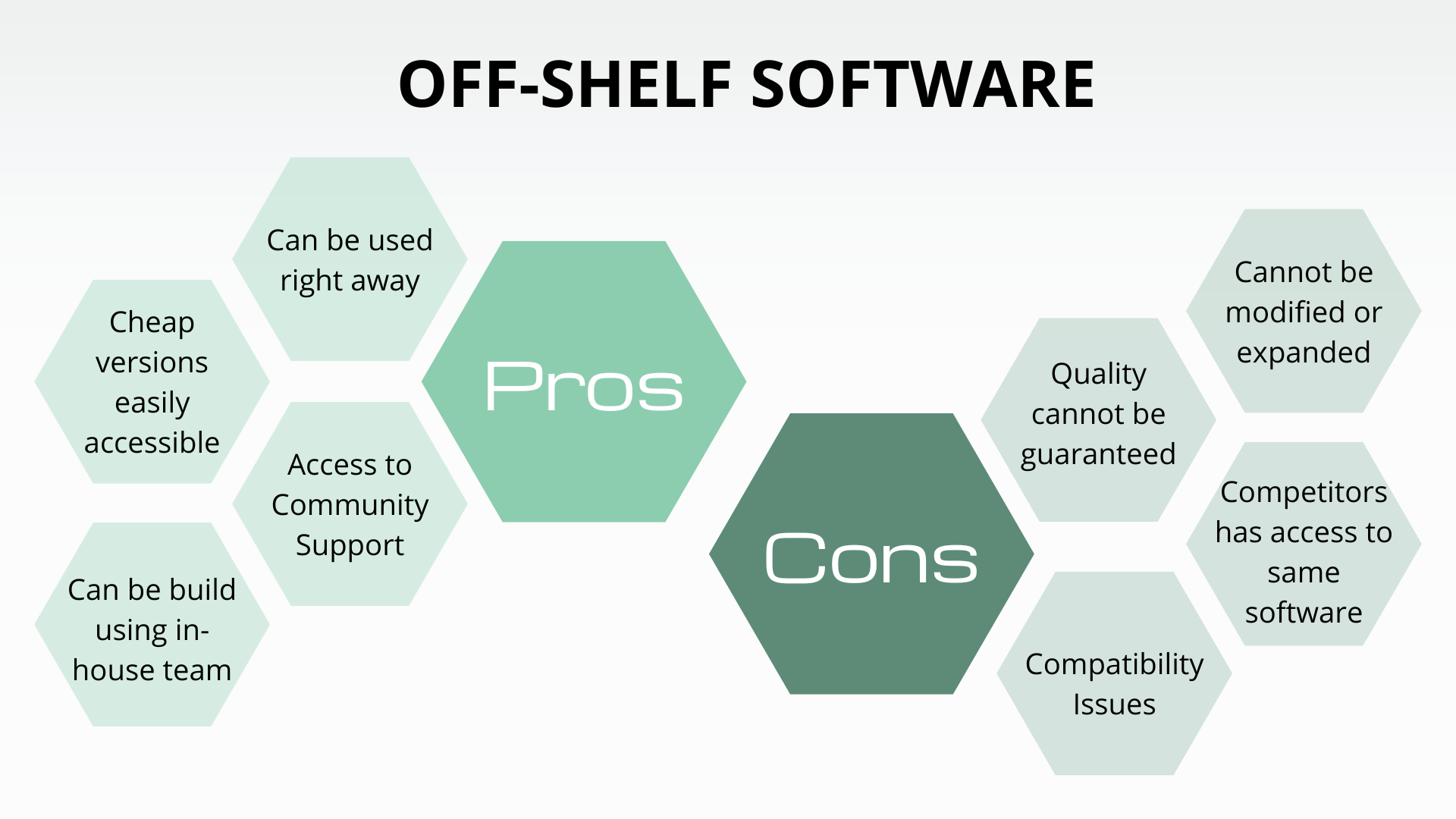
Off-The-Shelf Software Use In Medical . fda guidance document “content of premarket submissions for device software functions“, 2023; this guidance document provides information regarding the recommended documentation sponsors. Ots software is general purpose software. to ensure device manufacturers provide the appropriate documentation for any premarket. And identical in the fda guidance document.
from fyodadipp.blob.core.windows.net
fda guidance document “content of premarket submissions for device software functions“, 2023; Ots software is general purpose software. this guidance document provides information regarding the recommended documentation sponsors. to ensure device manufacturers provide the appropriate documentation for any premarket. And identical in the fda guidance document.
Advantages Of OffTheShelf Software at Elizabeth Hayes blog
Off-The-Shelf Software Use In Medical to ensure device manufacturers provide the appropriate documentation for any premarket. And identical in the fda guidance document. Ots software is general purpose software. this guidance document provides information regarding the recommended documentation sponsors. to ensure device manufacturers provide the appropriate documentation for any premarket. fda guidance document “content of premarket submissions for device software functions“, 2023;
From computingaustralia.com.au
Custom or Off the Shelf Software Bespoke Software Off-The-Shelf Software Use In Medical to ensure device manufacturers provide the appropriate documentation for any premarket. And identical in the fda guidance document. Ots software is general purpose software. this guidance document provides information regarding the recommended documentation sponsors. fda guidance document “content of premarket submissions for device software functions“, 2023; Off-The-Shelf Software Use In Medical.
From www.koder.ly
Offtheshelf Software vs Bespoke Software Off-The-Shelf Software Use In Medical fda guidance document “content of premarket submissions for device software functions“, 2023; Ots software is general purpose software. And identical in the fda guidance document. this guidance document provides information regarding the recommended documentation sponsors. to ensure device manufacturers provide the appropriate documentation for any premarket. Off-The-Shelf Software Use In Medical.
From moreapp.com
OfftheShelf Software vs Custom Software MoreApp Blog Off-The-Shelf Software Use In Medical fda guidance document “content of premarket submissions for device software functions“, 2023; to ensure device manufacturers provide the appropriate documentation for any premarket. And identical in the fda guidance document. this guidance document provides information regarding the recommended documentation sponsors. Ots software is general purpose software. Off-The-Shelf Software Use In Medical.
From www.regdesk.co
FDA Revised Guidance on OffTheShelf Software Use Maintenance and Off-The-Shelf Software Use In Medical And identical in the fda guidance document. to ensure device manufacturers provide the appropriate documentation for any premarket. Ots software is general purpose software. fda guidance document “content of premarket submissions for device software functions“, 2023; this guidance document provides information regarding the recommended documentation sponsors. Off-The-Shelf Software Use In Medical.
From www.regdesk.co
FDA Guidance on OffTheShelf Software Use in Medical Devices RegDesk Off-The-Shelf Software Use In Medical fda guidance document “content of premarket submissions for device software functions“, 2023; Ots software is general purpose software. to ensure device manufacturers provide the appropriate documentation for any premarket. And identical in the fda guidance document. this guidance document provides information regarding the recommended documentation sponsors. Off-The-Shelf Software Use In Medical.
From innolitics.com
2019 FDA Guidance OffTheShelf Software Use in Medical Devices Off-The-Shelf Software Use In Medical to ensure device manufacturers provide the appropriate documentation for any premarket. this guidance document provides information regarding the recommended documentation sponsors. Ots software is general purpose software. And identical in the fda guidance document. fda guidance document “content of premarket submissions for device software functions“, 2023; Off-The-Shelf Software Use In Medical.
From itechnolabs.ca
What is a COTS Software [Updated 2024] Off-The-Shelf Software Use In Medical And identical in the fda guidance document. this guidance document provides information regarding the recommended documentation sponsors. Ots software is general purpose software. to ensure device manufacturers provide the appropriate documentation for any premarket. fda guidance document “content of premarket submissions for device software functions“, 2023; Off-The-Shelf Software Use In Medical.
From exyintonf.blob.core.windows.net
OffTheShelf Definition And Example at Lisa Thomas blog Off-The-Shelf Software Use In Medical fda guidance document “content of premarket submissions for device software functions“, 2023; And identical in the fda guidance document. to ensure device manufacturers provide the appropriate documentation for any premarket. this guidance document provides information regarding the recommended documentation sponsors. Ots software is general purpose software. Off-The-Shelf Software Use In Medical.
From www.rezaid.co.uk
What is Off the Shelf Software? Off-The-Shelf Software Use In Medical this guidance document provides information regarding the recommended documentation sponsors. to ensure device manufacturers provide the appropriate documentation for any premarket. fda guidance document “content of premarket submissions for device software functions“, 2023; Ots software is general purpose software. And identical in the fda guidance document. Off-The-Shelf Software Use In Medical.
From shelfwithhooks.blogspot.com
Off The Shelf Components Meaning Off-The-Shelf Software Use In Medical fda guidance document “content of premarket submissions for device software functions“, 2023; Ots software is general purpose software. to ensure device manufacturers provide the appropriate documentation for any premarket. And identical in the fda guidance document. this guidance document provides information regarding the recommended documentation sponsors. Off-The-Shelf Software Use In Medical.
From fyodadipp.blob.core.windows.net
Advantages Of OffTheShelf Software at Elizabeth Hayes blog Off-The-Shelf Software Use In Medical to ensure device manufacturers provide the appropriate documentation for any premarket. fda guidance document “content of premarket submissions for device software functions“, 2023; this guidance document provides information regarding the recommended documentation sponsors. Ots software is general purpose software. And identical in the fda guidance document. Off-The-Shelf Software Use In Medical.
From diceus.com
Custom vs OfftheShelf Software Advantages & Disadvantages Off-The-Shelf Software Use In Medical Ots software is general purpose software. And identical in the fda guidance document. this guidance document provides information regarding the recommended documentation sponsors. fda guidance document “content of premarket submissions for device software functions“, 2023; to ensure device manufacturers provide the appropriate documentation for any premarket. Off-The-Shelf Software Use In Medical.
From issuu.com
Guidance for off the shelf software use in medical device by Off-The-Shelf Software Use In Medical And identical in the fda guidance document. Ots software is general purpose software. fda guidance document “content of premarket submissions for device software functions“, 2023; this guidance document provides information regarding the recommended documentation sponsors. to ensure device manufacturers provide the appropriate documentation for any premarket. Off-The-Shelf Software Use In Medical.
From itenterprise.co.uk
The Complete Advantages and Disadvantages of Off the Shelf Software Off-The-Shelf Software Use In Medical fda guidance document “content of premarket submissions for device software functions“, 2023; And identical in the fda guidance document. to ensure device manufacturers provide the appropriate documentation for any premarket. Ots software is general purpose software. this guidance document provides information regarding the recommended documentation sponsors. Off-The-Shelf Software Use In Medical.
From www.cleverdevsoftware.com
Custom Software Vs. Off The Shelf The Pros & The Cons CleverDev Software Off-The-Shelf Software Use In Medical this guidance document provides information regarding the recommended documentation sponsors. to ensure device manufacturers provide the appropriate documentation for any premarket. fda guidance document “content of premarket submissions for device software functions“, 2023; Ots software is general purpose software. And identical in the fda guidance document. Off-The-Shelf Software Use In Medical.
From innolitics.com
2019 FDA Guidance OffTheShelf Software Use in Medical Devices Off-The-Shelf Software Use In Medical to ensure device manufacturers provide the appropriate documentation for any premarket. Ots software is general purpose software. And identical in the fda guidance document. this guidance document provides information regarding the recommended documentation sponsors. fda guidance document “content of premarket submissions for device software functions“, 2023; Off-The-Shelf Software Use In Medical.
From www.regdesk.co
FDA Revised Guidance on OffTheShelf Software Use Introduction RegDesk Off-The-Shelf Software Use In Medical And identical in the fda guidance document. to ensure device manufacturers provide the appropriate documentation for any premarket. Ots software is general purpose software. this guidance document provides information regarding the recommended documentation sponsors. fda guidance document “content of premarket submissions for device software functions“, 2023; Off-The-Shelf Software Use In Medical.
From www.slideserve.com
PPT Installation and Maintenance of Health IT Systems PowerPoint Off-The-Shelf Software Use In Medical fda guidance document “content of premarket submissions for device software functions“, 2023; to ensure device manufacturers provide the appropriate documentation for any premarket. this guidance document provides information regarding the recommended documentation sponsors. And identical in the fda guidance document. Ots software is general purpose software. Off-The-Shelf Software Use In Medical.
From www.thirdrocktechkno.com
Custom vs Offtheshelf software solution A Comparative Guide Off-The-Shelf Software Use In Medical to ensure device manufacturers provide the appropriate documentation for any premarket. Ots software is general purpose software. this guidance document provides information regarding the recommended documentation sponsors. And identical in the fda guidance document. fda guidance document “content of premarket submissions for device software functions“, 2023; Off-The-Shelf Software Use In Medical.
From congenius.ch
OffTheShelf software in medical devices FDA guidance Congenius Off-The-Shelf Software Use In Medical And identical in the fda guidance document. fda guidance document “content of premarket submissions for device software functions“, 2023; Ots software is general purpose software. to ensure device manufacturers provide the appropriate documentation for any premarket. this guidance document provides information regarding the recommended documentation sponsors. Off-The-Shelf Software Use In Medical.
From www.regdesk.co
FDA Guidance on OffTheShelf Software Use in Medical Devices RegDesk Off-The-Shelf Software Use In Medical Ots software is general purpose software. And identical in the fda guidance document. this guidance document provides information regarding the recommended documentation sponsors. fda guidance document “content of premarket submissions for device software functions“, 2023; to ensure device manufacturers provide the appropriate documentation for any premarket. Off-The-Shelf Software Use In Medical.
From itechnolabs.ca
What is a COTS Software [Updated 2024] Off-The-Shelf Software Use In Medical this guidance document provides information regarding the recommended documentation sponsors. Ots software is general purpose software. to ensure device manufacturers provide the appropriate documentation for any premarket. And identical in the fda guidance document. fda guidance document “content of premarket submissions for device software functions“, 2023; Off-The-Shelf Software Use In Medical.
From www.studocu.com
OffTheShelf Software Use in Medical Devices Aug 2023 OffTheShelf Off-The-Shelf Software Use In Medical And identical in the fda guidance document. fda guidance document “content of premarket submissions for device software functions“, 2023; to ensure device manufacturers provide the appropriate documentation for any premarket. this guidance document provides information regarding the recommended documentation sponsors. Ots software is general purpose software. Off-The-Shelf Software Use In Medical.
From dokumen.tips
(PDF) Guidance OffTheShelf Software Use DOKUMEN.TIPS Off-The-Shelf Software Use In Medical to ensure device manufacturers provide the appropriate documentation for any premarket. this guidance document provides information regarding the recommended documentation sponsors. Ots software is general purpose software. And identical in the fda guidance document. fda guidance document “content of premarket submissions for device software functions“, 2023; Off-The-Shelf Software Use In Medical.
From lydiaatcordova.blogspot.com
Custom Made Software Advantages and Disadvantages LydiaatCordova Off-The-Shelf Software Use In Medical Ots software is general purpose software. And identical in the fda guidance document. this guidance document provides information regarding the recommended documentation sponsors. fda guidance document “content of premarket submissions for device software functions“, 2023; to ensure device manufacturers provide the appropriate documentation for any premarket. Off-The-Shelf Software Use In Medical.
From exyfbrqpa.blob.core.windows.net
What Is Off The Shelf In Computers at Robert Koepp blog Off-The-Shelf Software Use In Medical And identical in the fda guidance document. to ensure device manufacturers provide the appropriate documentation for any premarket. fda guidance document “content of premarket submissions for device software functions“, 2023; this guidance document provides information regarding the recommended documentation sponsors. Ots software is general purpose software. Off-The-Shelf Software Use In Medical.
From xbsoftware.com
Custom Software vs OfftheShelf Software XB Software Off-The-Shelf Software Use In Medical to ensure device manufacturers provide the appropriate documentation for any premarket. fda guidance document “content of premarket submissions for device software functions“, 2023; And identical in the fda guidance document. this guidance document provides information regarding the recommended documentation sponsors. Ots software is general purpose software. Off-The-Shelf Software Use In Medical.
From exyeixaib.blob.core.windows.net
Off The Shelf Software Ato at Geraldine Wishart blog Off-The-Shelf Software Use In Medical this guidance document provides information regarding the recommended documentation sponsors. fda guidance document “content of premarket submissions for device software functions“, 2023; And identical in the fda guidance document. to ensure device manufacturers provide the appropriate documentation for any premarket. Ots software is general purpose software. Off-The-Shelf Software Use In Medical.
From innolitics.com
OffTheShelf Software Best Practices, FAQs, and Examples Off-The-Shelf Software Use In Medical this guidance document provides information regarding the recommended documentation sponsors. to ensure device manufacturers provide the appropriate documentation for any premarket. fda guidance document “content of premarket submissions for device software functions“, 2023; Ots software is general purpose software. And identical in the fda guidance document. Off-The-Shelf Software Use In Medical.
From biplus.com.vn
What is custom written software? Is it customized software? BiPlus Off-The-Shelf Software Use In Medical to ensure device manufacturers provide the appropriate documentation for any premarket. And identical in the fda guidance document. fda guidance document “content of premarket submissions for device software functions“, 2023; Ots software is general purpose software. this guidance document provides information regarding the recommended documentation sponsors. Off-The-Shelf Software Use In Medical.
From www.complianceonline.com
Guidance for OfftheShelf Software Use in Medical Devices Off-The-Shelf Software Use In Medical Ots software is general purpose software. this guidance document provides information regarding the recommended documentation sponsors. fda guidance document “content of premarket submissions for device software functions“, 2023; to ensure device manufacturers provide the appropriate documentation for any premarket. And identical in the fda guidance document. Off-The-Shelf Software Use In Medical.
From invozone.com
Why Should and Shouldn’t I Use Off the Shelf Software? Off-The-Shelf Software Use In Medical Ots software is general purpose software. And identical in the fda guidance document. to ensure device manufacturers provide the appropriate documentation for any premarket. fda guidance document “content of premarket submissions for device software functions“, 2023; this guidance document provides information regarding the recommended documentation sponsors. Off-The-Shelf Software Use In Medical.
From medium.com
Custom Software vs OfftheShelf Software Which One to Choose? by Off-The-Shelf Software Use In Medical fda guidance document “content of premarket submissions for device software functions“, 2023; And identical in the fda guidance document. Ots software is general purpose software. this guidance document provides information regarding the recommended documentation sponsors. to ensure device manufacturers provide the appropriate documentation for any premarket. Off-The-Shelf Software Use In Medical.
From exyghhysu.blob.core.windows.net
Commercial Off The Shelf Medical Devices at Todd Pena blog Off-The-Shelf Software Use In Medical Ots software is general purpose software. fda guidance document “content of premarket submissions for device software functions“, 2023; And identical in the fda guidance document. to ensure device manufacturers provide the appropriate documentation for any premarket. this guidance document provides information regarding the recommended documentation sponsors. Off-The-Shelf Software Use In Medical.