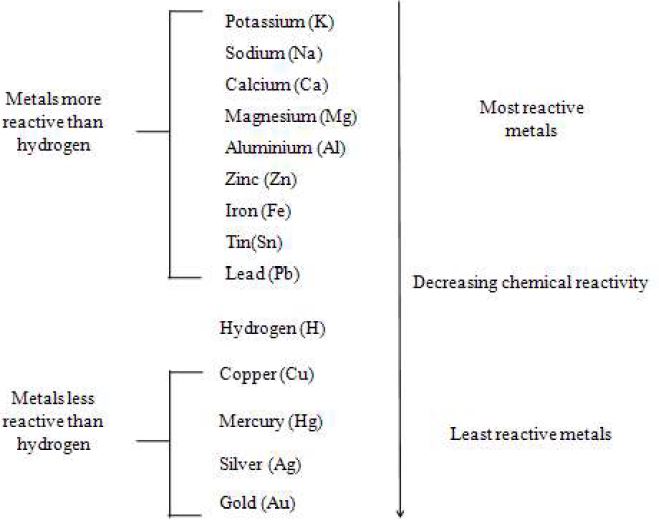
Zinc Copper And Magnesium In Order Of Reactivity . In order to determine the relative reactivity of each metal we will carry out displacement reactions by reacting. Magnesium > zinc > copper. the reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order of their reactivities. This is because magnesium could displace copper and zinc, zinc could only. In general, the more reactive a. zinc (zn) reacts with sulfuric acid (h 2 so 4) to produce zinc sulfate (znso 4) while liberating hydrogen gas (h 2). the reactivity series is a series of metals, in order of reactivity from highest to lowest. Zn (s) + h 2 so 4 (aq.) → znso 4 (aq.) + h 2 (g) 2. It is used to determine the products of. the order of reactivity is: The top metals are more reactive than the metals on the. copper, iron, zinc, and magnesium. 9 rows the reactivity series of metals is a chart listing metals in order of decreasing reactivity. the activity series is a chart of metals listed in order of declining relative reactivity.
from www.practically.com
9 rows the reactivity series of metals is a chart listing metals in order of decreasing reactivity. the order of reactivity is: In general, the more reactive a. The top metals are more reactive than the metals on the. zinc (zn) reacts with sulfuric acid (h 2 so 4) to produce zinc sulfate (znso 4) while liberating hydrogen gas (h 2). copper, iron, zinc, and magnesium. Magnesium > zinc > copper. In order to determine the relative reactivity of each metal we will carry out displacement reactions by reacting. the activity series is a chart of metals listed in order of declining relative reactivity. It is used to determine the products of.
Metals and Non Metals Practically Study Material
Zinc Copper And Magnesium In Order Of Reactivity Magnesium > zinc > copper. the reactivity series is a series of metals, in order of reactivity from highest to lowest. The top metals are more reactive than the metals on the. zinc (zn) reacts with sulfuric acid (h 2 so 4) to produce zinc sulfate (znso 4) while liberating hydrogen gas (h 2). Zn (s) + h 2 so 4 (aq.) → znso 4 (aq.) + h 2 (g) 2. 9 rows the reactivity series of metals is a chart listing metals in order of decreasing reactivity. the activity series is a chart of metals listed in order of declining relative reactivity. It is used to determine the products of. copper, iron, zinc, and magnesium. Magnesium > zinc > copper. the order of reactivity is: In general, the more reactive a. In order to determine the relative reactivity of each metal we will carry out displacement reactions by reacting. the reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order of their reactivities. This is because magnesium could displace copper and zinc, zinc could only.
From giogbvdps.blob.core.windows.net
Magnesium And Zinc Reactivity at Bruce Neal blog Zinc Copper And Magnesium In Order Of Reactivity Magnesium > zinc > copper. the order of reactivity is: The top metals are more reactive than the metals on the. In order to determine the relative reactivity of each metal we will carry out displacement reactions by reacting. zinc (zn) reacts with sulfuric acid (h 2 so 4) to produce zinc sulfate (znso 4) while liberating hydrogen. Zinc Copper And Magnesium In Order Of Reactivity.
From www.slideserve.com
PPT KS3 Chemistry PowerPoint Presentation, free download ID639260 Zinc Copper And Magnesium In Order Of Reactivity Zn (s) + h 2 so 4 (aq.) → znso 4 (aq.) + h 2 (g) 2. In order to determine the relative reactivity of each metal we will carry out displacement reactions by reacting. It is used to determine the products of. 9 rows the reactivity series of metals is a chart listing metals in order of decreasing. Zinc Copper And Magnesium In Order Of Reactivity.
From www.youtube.com
(a). Arrange the following metals in order of their chemical reactivity Zinc Copper And Magnesium In Order Of Reactivity copper, iron, zinc, and magnesium. the reactivity series is a series of metals, in order of reactivity from highest to lowest. The top metals are more reactive than the metals on the. In order to determine the relative reactivity of each metal we will carry out displacement reactions by reacting. zinc (zn) reacts with sulfuric acid (h. Zinc Copper And Magnesium In Order Of Reactivity.
From www.slideserve.com
PPT Magnesium reacts quicker than copper and iron. There is a league Zinc Copper And Magnesium In Order Of Reactivity In general, the more reactive a. zinc (zn) reacts with sulfuric acid (h 2 so 4) to produce zinc sulfate (znso 4) while liberating hydrogen gas (h 2). the activity series is a chart of metals listed in order of declining relative reactivity. copper, iron, zinc, and magnesium. Zn (s) + h 2 so 4 (aq.) →. Zinc Copper And Magnesium In Order Of Reactivity.
From brainly.in
What are reactivity series..? Brainly.in Zinc Copper And Magnesium In Order Of Reactivity It is used to determine the products of. the order of reactivity is: the activity series is a chart of metals listed in order of declining relative reactivity. The top metals are more reactive than the metals on the. the reactivity series is a series of metals, in order of reactivity from highest to lowest. Zn (s). Zinc Copper And Magnesium In Order Of Reactivity.
From dotandlinelearning.com
Chapter 9 Mastering Metal Science A Comprehensive Guide to IGCSE Zinc Copper And Magnesium In Order Of Reactivity the activity series is a chart of metals listed in order of declining relative reactivity. the order of reactivity is: Magnesium > zinc > copper. copper, iron, zinc, and magnesium. zinc (zn) reacts with sulfuric acid (h 2 so 4) to produce zinc sulfate (znso 4) while liberating hydrogen gas (h 2). In order to determine. Zinc Copper And Magnesium In Order Of Reactivity.
From askfilo.com
Below are some metals in decreasing order of reactivity.magnesiumzinci.. Zinc Copper And Magnesium In Order Of Reactivity the activity series is a chart of metals listed in order of declining relative reactivity. It is used to determine the products of. the reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order of their reactivities. Zn (s) + h 2 so 4 (aq.) → znso 4. Zinc Copper And Magnesium In Order Of Reactivity.
From www.gauthmath.com
Solved 14) The list gives the order of some metals and hydrogen in the Zinc Copper And Magnesium In Order Of Reactivity the reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order of their reactivities. copper, iron, zinc, and magnesium. the activity series is a chart of metals listed in order of declining relative reactivity. zinc (zn) reacts with sulfuric acid (h 2 so 4) to produce. Zinc Copper And Magnesium In Order Of Reactivity.
From www.teachoo.com
[Class 10] Keerti added dilute Hydrochloric acid to four metals and Zinc Copper And Magnesium In Order Of Reactivity Zn (s) + h 2 so 4 (aq.) → znso 4 (aq.) + h 2 (g) 2. It is used to determine the products of. Magnesium > zinc > copper. the order of reactivity is: The top metals are more reactive than the metals on the. zinc (zn) reacts with sulfuric acid (h 2 so 4) to produce. Zinc Copper And Magnesium In Order Of Reactivity.
From protonstalk.com
Electrochemical Series Features, Applications, Examples ProtonsTalk Zinc Copper And Magnesium In Order Of Reactivity This is because magnesium could displace copper and zinc, zinc could only. the activity series is a chart of metals listed in order of declining relative reactivity. In order to determine the relative reactivity of each metal we will carry out displacement reactions by reacting. zinc (zn) reacts with sulfuric acid (h 2 so 4) to produce zinc. Zinc Copper And Magnesium In Order Of Reactivity.
From revisechemistry.uk
Reactivity of Metals AQA C4 revisechemistry.uk Zinc Copper And Magnesium In Order Of Reactivity In order to determine the relative reactivity of each metal we will carry out displacement reactions by reacting. This is because magnesium could displace copper and zinc, zinc could only. the activity series is a chart of metals listed in order of declining relative reactivity. It is used to determine the products of. 9 rows the reactivity series. Zinc Copper And Magnesium In Order Of Reactivity.
From www.slideserve.com
PPT CHAPTER 3 METALS AND NON METALS PowerPoint Presentation, free Zinc Copper And Magnesium In Order Of Reactivity the reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order of their reactivities. In general, the more reactive a. The top metals are more reactive than the metals on the. the activity series is a chart of metals listed in order of declining relative reactivity. 9. Zinc Copper And Magnesium In Order Of Reactivity.
From polizneuro.weebly.com
Reactivity series of metals polizneuro Zinc Copper And Magnesium In Order Of Reactivity In order to determine the relative reactivity of each metal we will carry out displacement reactions by reacting. This is because magnesium could displace copper and zinc, zinc could only. the reactivity series is a series of metals, in order of reactivity from highest to lowest. 9 rows the reactivity series of metals is a chart listing metals. Zinc Copper And Magnesium In Order Of Reactivity.
From brainly.in
) Arrange these metals in decreasing order of their reactivity Zn, Al Zinc Copper And Magnesium In Order Of Reactivity It is used to determine the products of. the reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order of their reactivities. the activity series is a chart of metals listed in order of declining relative reactivity. the order of reactivity is: This is because magnesium could. Zinc Copper And Magnesium In Order Of Reactivity.
From mungfali.com
Reactivity Series Chart Zinc Copper And Magnesium In Order Of Reactivity Zn (s) + h 2 so 4 (aq.) → znso 4 (aq.) + h 2 (g) 2. the activity series is a chart of metals listed in order of declining relative reactivity. zinc (zn) reacts with sulfuric acid (h 2 so 4) to produce zinc sulfate (znso 4) while liberating hydrogen gas (h 2). the reactivity series. Zinc Copper And Magnesium In Order Of Reactivity.
From www.quora.com
Which is the most reactive metal out of zinc, copper, and magnesium Zinc Copper And Magnesium In Order Of Reactivity In order to determine the relative reactivity of each metal we will carry out displacement reactions by reacting. the activity series is a chart of metals listed in order of declining relative reactivity. The top metals are more reactive than the metals on the. It is used to determine the products of. Zn (s) + h 2 so 4. Zinc Copper And Magnesium In Order Of Reactivity.
From www.slideserve.com
PPT KS3 Chemistry PowerPoint Presentation, free download ID639260 Zinc Copper And Magnesium In Order Of Reactivity Magnesium > zinc > copper. copper, iron, zinc, and magnesium. the reactivity series is a series of metals, in order of reactivity from highest to lowest. the activity series is a chart of metals listed in order of declining relative reactivity. This is because magnesium could displace copper and zinc, zinc could only. the order of. Zinc Copper And Magnesium In Order Of Reactivity.
From knowt.com
Chapter 14 Reactivity series Flashcards Knowt Zinc Copper And Magnesium In Order Of Reactivity Zn (s) + h 2 so 4 (aq.) → znso 4 (aq.) + h 2 (g) 2. the activity series is a chart of metals listed in order of declining relative reactivity. Magnesium > zinc > copper. the reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order. Zinc Copper And Magnesium In Order Of Reactivity.
From www.slideserve.com
PPT Metal Reactions and Reactivity PowerPoint Presentation, free Zinc Copper And Magnesium In Order Of Reactivity the reactivity series is a series of metals, in order of reactivity from highest to lowest. Zn (s) + h 2 so 4 (aq.) → znso 4 (aq.) + h 2 (g) 2. In order to determine the relative reactivity of each metal we will carry out displacement reactions by reacting. This is because magnesium could displace copper and. Zinc Copper And Magnesium In Order Of Reactivity.
From www.hanlin.com
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:2.4.1 Metals Reacting with Zinc Copper And Magnesium In Order Of Reactivity Magnesium > zinc > copper. zinc (zn) reacts with sulfuric acid (h 2 so 4) to produce zinc sulfate (znso 4) while liberating hydrogen gas (h 2). It is used to determine the products of. Zn (s) + h 2 so 4 (aq.) → znso 4 (aq.) + h 2 (g) 2. The top metals are more reactive than. Zinc Copper And Magnesium In Order Of Reactivity.
From giobcjzxq.blob.core.windows.net
Which Is The Least Active Metal at Phillip Nagle blog Zinc Copper And Magnesium In Order Of Reactivity the reactivity series is a series of metals, in order of reactivity from highest to lowest. Magnesium > zinc > copper. zinc (zn) reacts with sulfuric acid (h 2 so 4) to produce zinc sulfate (znso 4) while liberating hydrogen gas (h 2). In order to determine the relative reactivity of each metal we will carry out displacement. Zinc Copper And Magnesium In Order Of Reactivity.
From www.adda247.com
Reactivity series of Metals & Non Metals For Class 10 Zinc Copper And Magnesium In Order Of Reactivity The top metals are more reactive than the metals on the. the activity series is a chart of metals listed in order of declining relative reactivity. This is because magnesium could displace copper and zinc, zinc could only. the order of reactivity is: zinc (zn) reacts with sulfuric acid (h 2 so 4) to produce zinc sulfate. Zinc Copper And Magnesium In Order Of Reactivity.
From www.geeksforgeeks.org
Reactivity Series Reactivity of Metals, Features, Tricks Zinc Copper And Magnesium In Order Of Reactivity This is because magnesium could displace copper and zinc, zinc could only. In order to determine the relative reactivity of each metal we will carry out displacement reactions by reacting. the order of reactivity is: the activity series is a chart of metals listed in order of declining relative reactivity. In general, the more reactive a. The top. Zinc Copper And Magnesium In Order Of Reactivity.
From www.teachoo.com
Reactivity Series of Metals Chart [and How to remember] Teachoo Zinc Copper And Magnesium In Order Of Reactivity zinc (zn) reacts with sulfuric acid (h 2 so 4) to produce zinc sulfate (znso 4) while liberating hydrogen gas (h 2). The top metals are more reactive than the metals on the. the reactivity series is a series of metals, in order of reactivity from highest to lowest. Zn (s) + h 2 so 4 (aq.) →. Zinc Copper And Magnesium In Order Of Reactivity.
From sciencenotes.org
Activity Series of Metals (Reactivity Series) Zinc Copper And Magnesium In Order Of Reactivity the reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order of their reactivities. The top metals are more reactive than the metals on the. Zn (s) + h 2 so 4 (aq.) → znso 4 (aq.) + h 2 (g) 2. copper, iron, zinc, and magnesium. This. Zinc Copper And Magnesium In Order Of Reactivity.
From byjus.com
Sort the following metals in the increasing order of their reactivity. Zinc Copper And Magnesium In Order Of Reactivity It is used to determine the products of. Zn (s) + h 2 so 4 (aq.) → znso 4 (aq.) + h 2 (g) 2. Magnesium > zinc > copper. the activity series is a chart of metals listed in order of declining relative reactivity. the order of reactivity is: zinc (zn) reacts with sulfuric acid (h. Zinc Copper And Magnesium In Order Of Reactivity.
From www.practically.com
Metals and Non Metals Practically Study Material Zinc Copper And Magnesium In Order Of Reactivity This is because magnesium could displace copper and zinc, zinc could only. the reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order of their reactivities. the activity series is a chart of metals listed in order of declining relative reactivity. Magnesium > zinc > copper. Zn (s). Zinc Copper And Magnesium In Order Of Reactivity.
From www.numerade.com
SOLVED 'Which row gives the correct order of reactivity of the four Zinc Copper And Magnesium In Order Of Reactivity In general, the more reactive a. Magnesium > zinc > copper. The top metals are more reactive than the metals on the. copper, iron, zinc, and magnesium. Zn (s) + h 2 so 4 (aq.) → znso 4 (aq.) + h 2 (g) 2. the reactivity series is a series of metals, in order of reactivity from highest. Zinc Copper And Magnesium In Order Of Reactivity.
From brainly.in
1Metallic oxides of zinc, magnesium and copper were beated with Zinc Copper And Magnesium In Order Of Reactivity In order to determine the relative reactivity of each metal we will carry out displacement reactions by reacting. It is used to determine the products of. In general, the more reactive a. copper, iron, zinc, and magnesium. The top metals are more reactive than the metals on the. the reactivity series of metals, also known as the activity. Zinc Copper And Magnesium In Order Of Reactivity.
From www.slideserve.com
PPT METAL REACTIONS PowerPoint Presentation, free download ID2670563 Zinc Copper And Magnesium In Order Of Reactivity the reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order of their reactivities. the reactivity series is a series of metals, in order of reactivity from highest to lowest. In general, the more reactive a. 9 rows the reactivity series of metals is a chart listing. Zinc Copper And Magnesium In Order Of Reactivity.
From www.crestolympiads.com
Metals and Nonmetals Notes Science Olympiad Class 8 Zinc Copper And Magnesium In Order Of Reactivity 9 rows the reactivity series of metals is a chart listing metals in order of decreasing reactivity. zinc (zn) reacts with sulfuric acid (h 2 so 4) to produce zinc sulfate (znso 4) while liberating hydrogen gas (h 2). In order to determine the relative reactivity of each metal we will carry out displacement reactions by reacting. . Zinc Copper And Magnesium In Order Of Reactivity.
From getrevising.co.uk
The Reactivity Series Revision Notes in GCSE Chemistry Zinc Copper And Magnesium In Order Of Reactivity the reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order of their reactivities. Zn (s) + h 2 so 4 (aq.) → znso 4 (aq.) + h 2 (g) 2. This is because magnesium could displace copper and zinc, zinc could only. the order of reactivity is:. Zinc Copper And Magnesium In Order Of Reactivity.
From www.compoundchem.com
The Metal Reactivity Series Compound Interest Zinc Copper And Magnesium In Order Of Reactivity copper, iron, zinc, and magnesium. zinc (zn) reacts with sulfuric acid (h 2 so 4) to produce zinc sulfate (znso 4) while liberating hydrogen gas (h 2). Zn (s) + h 2 so 4 (aq.) → znso 4 (aq.) + h 2 (g) 2. 9 rows the reactivity series of metals is a chart listing metals in. Zinc Copper And Magnesium In Order Of Reactivity.
From mrcartlidgesaigon.edublogs.org
C10 Metals Science) Mr Cartlidge's second Science Blog Zinc Copper And Magnesium In Order Of Reactivity the reactivity series is a series of metals, in order of reactivity from highest to lowest. The top metals are more reactive than the metals on the. In order to determine the relative reactivity of each metal we will carry out displacement reactions by reacting. This is because magnesium could displace copper and zinc, zinc could only. zinc. Zinc Copper And Magnesium In Order Of Reactivity.
From blog.thepipingmart.com
Metallic Oxides of Zinc, Magnesium, and Copper Zinc Copper And Magnesium In Order Of Reactivity The top metals are more reactive than the metals on the. the order of reactivity is: copper, iron, zinc, and magnesium. the reactivity series is a series of metals, in order of reactivity from highest to lowest. In order to determine the relative reactivity of each metal we will carry out displacement reactions by reacting. Zn (s). Zinc Copper And Magnesium In Order Of Reactivity.