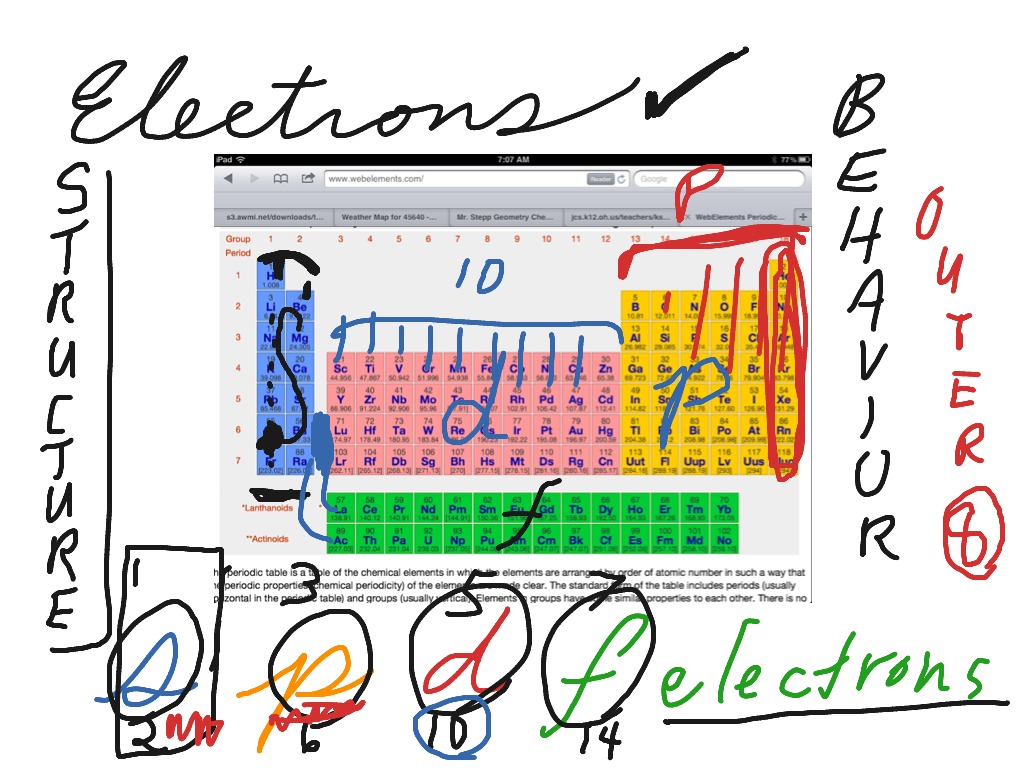
Full Meaning Of S P D F In Chemistry . How many d orbitals must be. The four basic types of orbitals are designated as s, p, d, and f. how would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals? this page explains what atomic orbitals are in a way that makes them understandable for introductory courses such as uk a level and. Within each shell of an atom there are. an atomic orbital is the probability description of where an electron can be found. The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the. 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). what does s, p, d, f stand for? the s, p, d, and f, respectively stand for sharp, primary, diffuse and fundamental. How many 2p orbitals are there in an atom?
from utedzz.blogspot.com
How many 2p orbitals are there in an atom? what does s, p, d, f stand for? the s, p, d, and f, respectively stand for sharp, primary, diffuse and fundamental. How many d orbitals must be. The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the. this page explains what atomic orbitals are in a way that makes them understandable for introductory courses such as uk a level and. how would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals? an atomic orbital is the probability description of where an electron can be found. The four basic types of orbitals are designated as s, p, d, and f. 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental).
Periodic Table Blocks S P D F Periodic Table Timeline
Full Meaning Of S P D F In Chemistry the s, p, d, and f, respectively stand for sharp, primary, diffuse and fundamental. what does s, p, d, f stand for? the s, p, d, and f, respectively stand for sharp, primary, diffuse and fundamental. 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). how would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals? The four basic types of orbitals are designated as s, p, d, and f. The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the. How many 2p orbitals are there in an atom? an atomic orbital is the probability description of where an electron can be found. How many d orbitals must be. this page explains what atomic orbitals are in a way that makes them understandable for introductory courses such as uk a level and. Within each shell of an atom there are.
From www.youtube.com
s, p, d, f Block Elements Periodic Table L3 NEET Chemistry YouTube Full Meaning Of S P D F In Chemistry an atomic orbital is the probability description of where an electron can be found. the s, p, d, and f, respectively stand for sharp, primary, diffuse and fundamental. How many 2p orbitals are there in an atom? The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the. . Full Meaning Of S P D F In Chemistry.
From utedzz.blogspot.com
Modern Periodic Table S P D F Blocks Periodic Table Timeline Full Meaning Of S P D F In Chemistry Within each shell of an atom there are. how would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals? How many d orbitals must be. this page explains what atomic orbitals are in a way that makes them understandable for introductory courses such as uk a level and. an atomic orbital is. Full Meaning Of S P D F In Chemistry.
From www.youtube.com
TIPS FOR ELECTRONIC CONFIGURATION│ 10 ICSE CHEMISTRY PERIODIC TABLE │S Full Meaning Of S P D F In Chemistry the s, p, d, and f, respectively stand for sharp, primary, diffuse and fundamental. The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the. How many d orbitals must be. an atomic orbital is the probability description of where an electron can be found. what does s,. Full Meaning Of S P D F In Chemistry.
From h-o-m-e.org
Chemistry Sublevels An Introduction to s, p, d, and f Sublevels Full Meaning Of S P D F In Chemistry How many d orbitals must be. this page explains what atomic orbitals are in a way that makes them understandable for introductory courses such as uk a level and. How many 2p orbitals are there in an atom? 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp,. Full Meaning Of S P D F In Chemistry.
From lavelle.chem.ucla.edu
s,p,d,f orbital characteristics? CHEMISTRY COMMUNITY Full Meaning Of S P D F In Chemistry how would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals? 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). an atomic orbital is the probability description of where an electron can be found. the s,. Full Meaning Of S P D F In Chemistry.
From utedzz.blogspot.com
Periodic Table Blocks S P D F Periodic Table Timeline Full Meaning Of S P D F In Chemistry The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the. what does s, p, d, f stand for? The four basic types of orbitals are designated as s, p, d, and f. how would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals?. Full Meaning Of S P D F In Chemistry.
From stock.adobe.com
Periodic table. s,p,d,f block elements. Electron configuration Full Meaning Of S P D F In Chemistry the s, p, d, and f, respectively stand for sharp, primary, diffuse and fundamental. The four basic types of orbitals are designated as s, p, d, and f. what does s, p, d, f stand for? How many 2p orbitals are there in an atom? how would you describe the shapes and relative energies of the s,p,d,. Full Meaning Of S P D F In Chemistry.
From scienceline.org
The FBlock An introduction Scienceline Full Meaning Of S P D F In Chemistry Within each shell of an atom there are. How many d orbitals must be. 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). this page explains what atomic orbitals are in a way that makes them understandable for introductory courses such as uk. Full Meaning Of S P D F In Chemistry.
From www.pinterest.com
A CHART of the spdf ELECTRON ORBITALS Chemistry education, Teaching Full Meaning Of S P D F In Chemistry an atomic orbital is the probability description of where an electron can be found. The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the. How many 2p orbitals are there in an atom? the s, p, d, and f, respectively stand for sharp, primary, diffuse and fundamental. Within. Full Meaning Of S P D F In Chemistry.
From www.vrogue.co
A Chart Of The Spdf Electron Orbitals Chemistry Educa vrogue.co Full Meaning Of S P D F In Chemistry The four basic types of orbitals are designated as s, p, d, and f. How many d orbitals must be. an atomic orbital is the probability description of where an electron can be found. The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the. how would you describe. Full Meaning Of S P D F In Chemistry.
From general.chemistrysteps.com
s, p, d, f Atomic Orbitals Chemistry Steps Full Meaning Of S P D F In Chemistry How many d orbitals must be. the s, p, d, and f, respectively stand for sharp, primary, diffuse and fundamental. 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). an atomic orbital is the probability description of where an electron can be. Full Meaning Of S P D F In Chemistry.
From www.youtube.com
Electron configuration spdf notation Part 2 YouTube Full Meaning Of S P D F In Chemistry How many d orbitals must be. The four basic types of orbitals are designated as s, p, d, and f. how would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals? The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the. an atomic. Full Meaning Of S P D F In Chemistry.
From wisc.pb.unizin.org
Chapter 2 Electrons and Atoms Chemistry 109 Full Meaning Of S P D F In Chemistry an atomic orbital is the probability description of where an electron can be found. The four basic types of orbitals are designated as s, p, d, and f. How many 2p orbitals are there in an atom? Within each shell of an atom there are. 12 rows there are four types of orbitals that you should be familiar. Full Meaning Of S P D F In Chemistry.
From general.chemistrysteps.com
s, p, d, f Atomic Orbitals Chemistry Steps Full Meaning Of S P D F In Chemistry this page explains what atomic orbitals are in a way that makes them understandable for introductory courses such as uk a level and. 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). The four basic types of orbitals are designated as s, p,. Full Meaning Of S P D F In Chemistry.
From www.studocu.com
S,P,D,F Block and Hydrogen bonds in chemistry/ B Sc chemistry /Kerala Full Meaning Of S P D F In Chemistry The four basic types of orbitals are designated as s, p, d, and f. 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). Within each shell of an atom there are. how would you describe the shapes and relative energies of the s,p,d,. Full Meaning Of S P D F In Chemistry.
From www.youtube.com
Electron configuration spdf notation Part 1 YouTube Full Meaning Of S P D F In Chemistry How many 2p orbitals are there in an atom? The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the. How many d orbitals must be. the s, p, d, and f, respectively stand for sharp, primary, diffuse and fundamental. what does s, p, d, f stand for? . Full Meaning Of S P D F In Chemistry.
From socratic.org
s,p,d,f Orbitals Chemistry Socratic Full Meaning Of S P D F In Chemistry this page explains what atomic orbitals are in a way that makes them understandable for introductory courses such as uk a level and. How many 2p orbitals are there in an atom? The four basic types of orbitals are designated as s, p, d, and f. The orbital names s, p, d, and f stand for names given to. Full Meaning Of S P D F In Chemistry.
From byjus.com
What is the value of s,p,d.f? Full Meaning Of S P D F In Chemistry the s, p, d, and f, respectively stand for sharp, primary, diffuse and fundamental. The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the. How many d orbitals must be. Within each shell of an atom there are. how would you describe the shapes and relative energies of. Full Meaning Of S P D F In Chemistry.
From www.pinterest.com
s, p, d and fBlock Elements Chemistry notes, Chemistry basics Full Meaning Of S P D F In Chemistry The four basic types of orbitals are designated as s, p, d, and f. how would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals? what does s, p, d, f stand for? How many 2p orbitals are there in an atom? this page explains what atomic orbitals are in a way. Full Meaning Of S P D F In Chemistry.
From www.pinterest.com
What Do S, P, D, and F Mean in Chemistry, and Why Do You Need Them Full Meaning Of S P D F In Chemistry what does s, p, d, f stand for? The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the. the s, p, d, and f, respectively stand for sharp, primary, diffuse and fundamental. this page explains what atomic orbitals are in a way that makes them understandable for. Full Meaning Of S P D F In Chemistry.
From ar.inspiredpencil.com
S P D F Orbital Full Meaning Of S P D F In Chemistry how would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals? The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the. the s, p, d, and f, respectively stand for sharp, primary, diffuse and fundamental. The four basic types of orbitals are designated. Full Meaning Of S P D F In Chemistry.
From www.youtube.com
🙏Modern Periodic Table Atomic Number Structure Size s p d f Name Size 🙏 Full Meaning Of S P D F In Chemistry how would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals? what does s, p, d, f stand for? this page explains what atomic orbitals are in a way that makes them understandable for introductory courses such as uk a level and. the s, p, d, and f, respectively stand for. Full Meaning Of S P D F In Chemistry.
From www.youtube.com
how to learn periodic table easily.s p d f blocks basics.groups,periods Full Meaning Of S P D F In Chemistry what does s, p, d, f stand for? the s, p, d, and f, respectively stand for sharp, primary, diffuse and fundamental. Within each shell of an atom there are. 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). How many d. Full Meaning Of S P D F In Chemistry.
From es.dreamstime.com
Tabla Periódica De Los Elementos Coloreados Según Su Bloque S P D F Full Meaning Of S P D F In Chemistry an atomic orbital is the probability description of where an electron can be found. what does s, p, d, f stand for? 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). the s, p, d, and f, respectively stand for sharp,. Full Meaning Of S P D F In Chemistry.
From chemwiki.ucdavis.edu
Electron Configuration of Transition Metals Chemwiki Full Meaning Of S P D F In Chemistry 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). Within each shell of an atom there are. The four basic types of orbitals are designated as s, p, d, and f. The orbital names s, p, d, and f stand for names given to. Full Meaning Of S P D F In Chemistry.
From www.youtube.com
Electron Configuration What is it? What are S P D F subshells? How to Full Meaning Of S P D F In Chemistry How many 2p orbitals are there in an atom? The four basic types of orbitals are designated as s, p, d, and f. what does s, p, d, f stand for? this page explains what atomic orbitals are in a way that makes them understandable for introductory courses such as uk a level and. How many d orbitals. Full Meaning Of S P D F In Chemistry.
From abacus.bates.edu
Chem Notes Full Meaning Of S P D F In Chemistry the s, p, d, and f, respectively stand for sharp, primary, diffuse and fundamental. an atomic orbital is the probability description of where an electron can be found. How many d orbitals must be. what does s, p, d, f stand for? The four basic types of orbitals are designated as s, p, d, and f. Within. Full Meaning Of S P D F In Chemistry.
From www.toppr.com
Write the general electronic configuration of s, p, d and fblock elements. Full Meaning Of S P D F In Chemistry How many 2p orbitals are there in an atom? How many d orbitals must be. The four basic types of orbitals are designated as s, p, d, and f. the s, p, d, and f, respectively stand for sharp, primary, diffuse and fundamental. an atomic orbital is the probability description of where an electron can be found. Within. Full Meaning Of S P D F In Chemistry.
From ar.inspiredpencil.com
Electron Shells S P D F Full Meaning Of S P D F In Chemistry the s, p, d, and f, respectively stand for sharp, primary, diffuse and fundamental. The four basic types of orbitals are designated as s, p, d, and f. 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). The orbital names s, p, d,. Full Meaning Of S P D F In Chemistry.
From www.vrogue.co
A Chart Of The Spdf Electron Orbitals Chemistry Educa vrogue.co Full Meaning Of S P D F In Chemistry How many 2p orbitals are there in an atom? this page explains what atomic orbitals are in a way that makes them understandable for introductory courses such as uk a level and. The four basic types of orbitals are designated as s, p, d, and f. How many d orbitals must be. 12 rows there are four types. Full Meaning Of S P D F In Chemistry.
From www.youtube.com
d and f block elements shape of p orbitals s,p,d,f orbital By Full Meaning Of S P D F In Chemistry this page explains what atomic orbitals are in a way that makes them understandable for introductory courses such as uk a level and. what does s, p, d, f stand for? how would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals? The four basic types of orbitals are designated as s,. Full Meaning Of S P D F In Chemistry.
From bilifforyou.blogspot.com
Why are the orbitals shells called s, p, d, f, etc.? Is there a reason? Full Meaning Of S P D F In Chemistry How many d orbitals must be. 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). The four basic types of orbitals are designated as s, p, d, and f. what does s, p, d, f stand for? How many 2p orbitals are there. Full Meaning Of S P D F In Chemistry.
From www.youtube.com
Electronic Configuration Easy Trick (s,p,d,f), Sequence of Atomic Full Meaning Of S P D F In Chemistry the s, p, d, and f, respectively stand for sharp, primary, diffuse and fundamental. The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the. How many 2p orbitals are there in an atom? 12 rows there are four types of orbitals that you should be familiar with s,. Full Meaning Of S P D F In Chemistry.
From general.chemistrysteps.com
s, p, d, f Atomic Orbitals Chemistry Steps Full Meaning Of S P D F In Chemistry an atomic orbital is the probability description of where an electron can be found. how would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals? what does s, p, d, f stand for? How many 2p orbitals are there in an atom? Within each shell of an atom there are. 12. Full Meaning Of S P D F In Chemistry.
From www.youtube.com
L4 Shapes of s, p, d, f orbitals bsc 4th sem chemistry Quantum Full Meaning Of S P D F In Chemistry 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). an atomic orbital is the probability description of where an electron can be found. the s, p, d, and f, respectively stand for sharp, primary, diffuse and fundamental. The four basic types of. Full Meaning Of S P D F In Chemistry.